r-stability-data-thermal-stress-cycling
Dieses Dokument ist Teil der Anfrage „Comirnaty Einfrieren Auftauen Sicherheit Impfstoff“
090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT) BNT162b2 3.2.P.8.3. Stability Data Thermal — Stress and Cycling TABLE OF CONTENTS LIST OF TABLES ............csaussnessnsssnsnonsonnennennennennnnontnnesnnnsnnennnsnnenesnnnnnnsonnennennennennorsnonsonsneneane 3.2.P.8.3. STABILITY DATA - THERMAL - STRESS AND CYCLING ........unsenenesene CONFIDENTIAL Page l

090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT) BNT162b2 3.2.P.8.3. Stability Data Thermal — Stress and Cycling LIST OF TABLES Table 3.2.P.8.3-1. Summary of Drug Product Thermal Stability Studies ...................... Table 3.2.P.8.3-2. Stability Data for Drug Product PPQ Lot EL8723 Stored at 25 +2 °C/60 + 5% RH (Pfizer, Puurs).......uusceeseeseesesneneesnenenensnennnnnnnnnnennennnnn Table 3.2.P.8.3-3. Stability Data for Drug Product PPQ Lot EL8723 Stored at 30 +2 °C/65 # 5% RH (Pfizer, Puurs)...............u0u0n0sennennnesnennnnnnenennennn nenn Table 3.2.P.8.3-4. Stability Data for Drug Product Emergency Supply Batch EJ1688 Stored at 25 +2 °C/60 # 5% RH .........unsersersernersenenennnnnnnnnnennnnnnnnnnnnn Table 3.2.P.8.3-5. Stability Data for Drug Product Emergency Supply Batch EK1768 Stored at 25 +2 °C/60 # 5% RH............cunsenssnsennsnnnnonsnnnsnnnnnnnnnnenennnn Table 3.2.P.8.3-6. Stability Data for Drug Product Emergency Supply Batch EJ1686 Stored at 25 +2 °C/60 + 5% RH............cunsnesnnennennnnnnnnnsonnnnnnnne nenn Table 3.2.P.8.3-7. Stability Data for Drug Product Emergency Supply Batch EJ1685 Stored at 25 +2 °C/60 + 5% RH............cunsnesnnennsnnnnonnnnsonnnnnnnnnennenn Table 3.2.P.8.3-8. Stability Data for Drug Product Emergency Supply Batch EJ0553 Stored at 25 +2 °C/60 + 5% RH............curs0ueeneennennennnnnnnnonnnnnnnnen nenn Table 3.2.P.8.3-9. Stability Data for Drug Product Emergency Supply Lot EE8493 Stored at 25 +2 °C/60 + 5% RH.........n.cunsenssnnennsnnnnnennnnonnennnnnennnenn Table 3.2.P.8.3-10. Stability Data for Drug Product Emergency Supply Lot EE8493 Stored 30+2 °C/65 +5 RH ........eneceesenssnnennennennnnnnnnonnnnnnnnennnnnnn Table 3.2.P.8.3-11. Stability Data for Drug Product PPQ Lot EL3248 Stored at 25 +2 °C/60 + 5% RH (Pfizer, Kalamazoo) ............cuu2cuussonsennnennnenseneennennnnne Table 3.2.P.8.3-12. Stability Data for Drug Product PPQ Lot EL3248 Stored at 30 +2 °C/65 + 5% RH (Pfizer, Kalamazoo) ...........ccueesesesneesnnnennnnnnnnnenennnnn Table 3.2.P.8.3-13. Stability Data for Drug Product Emergency Supply Batch EH9899 Stored at 25 +2 °C/60 # 5% RH .........rscnssnesneennennnnnnennnsnnennnennnennnne Table 3.2.P.8.3-14. Stability Data for Polymun Scientific Drug Product BNT162b2 Lot BCV40420-A Stored at 25 &2 °C ...nnnnnersnssnrsonsnnnnnenennnnenonsnnnnnsnnenenn Table 3.2.P.8.3-15. Stability Data for Polymun Scientific Drug Product BNT162b1 Lot BCV10320-A Stored at 25 £2 °C ....nnesessesesersnnnennnnsnnnnennnnnnnnen nennen Table 3.2.P.8.3-16. Thermal Cycling Stability Data for Drug Product Emergency Supply Batch EK1768 .....uueseesessenesenersnenneneeneonensonenenenensnnnnsonnensnnnenenn Table 3.2.P.8.3-17. Thermal Cycling Stability Data for Drug Product Emergency Supply Batch EJ1686.......uuenneseneensesnesnennennennnonnnnnnnennennnnnnennnennnonsenann Table 3.2.P.8.3-18. Thermal Cycling Stability Data for Drug Product PPQ Lot EN1195 Table 3.2.P.8.3-19. Thermal Cycling Stability Data for Drug Product PPQ Lot EL8723 Table 3.2.P.8.3-20. Thermal Cycling Stability Data for Drug Product PPQ Lot EK4242 CONFIDENTIAL Page 2

090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT) BNT162b2 3.2.P.8.3. Stability Data Thermal — Stress and Cycling Table 3.2.P.8.3-21. Thermal Cycling Stability Data for Drug Product PPQ Lot EL7834....... 29 Table 3.2.P.8.3-22. Thermal Cycling Stability Data for Drug Product PPQ Lot EL9266........ 31 Table 3.2.P.8.3-23. Thermal Cycling Stability Data for Drug Product PPQ Lot EL3249.......33 Table 3.2.P.8.3-24. Thermal Cycling Stability Data for Drug Product PPQ Lot EL8723 (-20 45 °C) eeseenersnensnennennenensnenennensnnenneenenensnenenennensensnnnnsensensenennensesonnensonanen 35 Table 3.2.P.8.3-25. Thermal Cycling Stability Data for Drug Product PPQ Lot EL8723 (210 8 °C) ...ueseesesnennenusnnnnenennnnnesnnenenennennennennenennsnansesnnnnennsunssenssnenensnnansenannn 37 Table 3.2.P.8.3-26. Freeze Thaw Cycling Stability Data for Drug Product PPQ Lot EL8723 ...neessesessesesnnenenesnennennenennennennenensnnansennnnanensnnnenennonsenennonsesonnansenanen 38 Table 3.2.P.8.3-27. Thermal Cycling Stability Data for Drug Product PPQ Lot ET0384....... 39 CONFIDENTIAL Page 3

090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT) BNT162b2 3.2.P.8.3. Stability Data Thermal — Stress and Cycling 3.2.P.8.3. STABILITY DATA - THERMAL - STRESS AND CYCLING Data from stability studies on BNT162b2 drug product lots stored at the thermal stress conditions of 25 + 2 °C/60 + 5% RH and 30 + 2 °C/65 + 5% RH, as well as thermal cycling studies, are presented for emergency supply and process performance qualification lots manufactured by Polymun Scientific (with fill and finish at Pfizer, Puurs), mibe (with fill and finish at Pfizer, Puurs) and Pfizer, Puurs. Additionally, data from supportive stability studies for one clinical BNT162b2 drug product lot and one clinical supportive BNT162b1 drug product lot stored at the thermal stress condition of 25 + 2 °C and manufactured by Polymun Scientific is also presented. All studies are listed in Table 3.2.P.8.3-1. Results will be provided in Table 3.2.P.8.3-2 through Table 3.2.P.8.3-27. CONFIDENTIAL Page 4

090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT)
BNT162b2
3.2.P.8.3. Stability Data
Thermal — Stress and Cycling
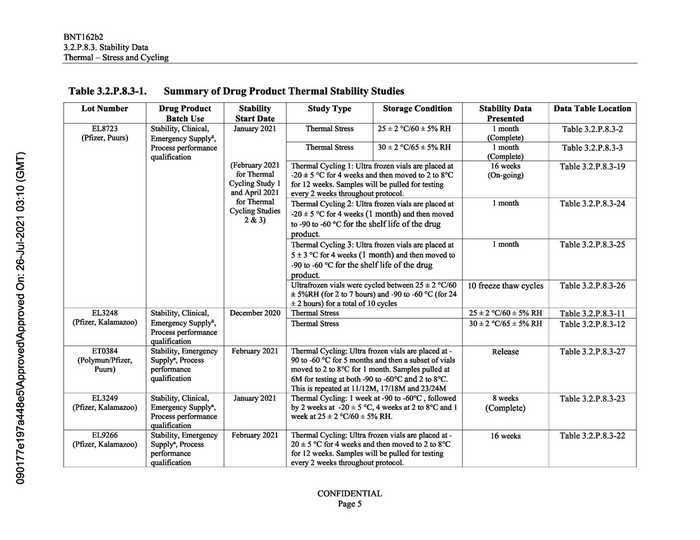
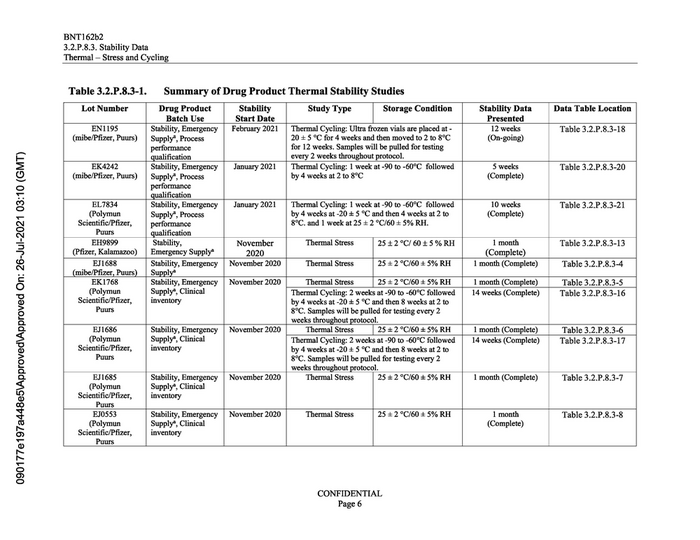
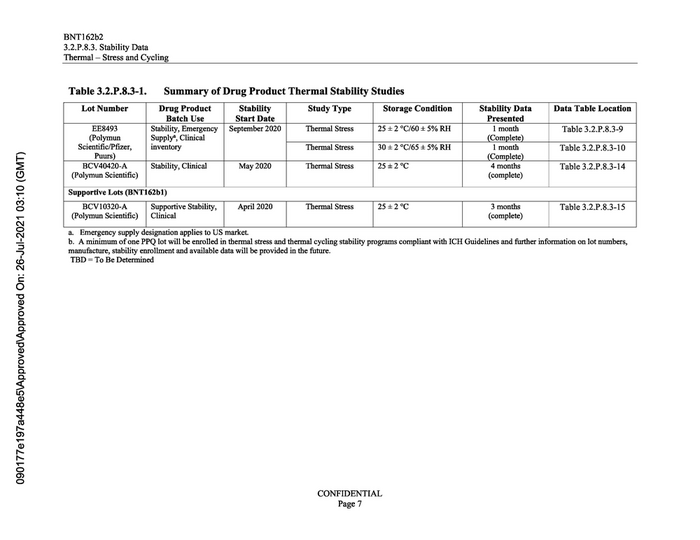
Table 3.2.P.8.3-1.
Summary of Drug Product Thermal Stability Studies
(Pfizer, Kalamazoo)
Supply”, Process
performance
qualification
20 #5 °C for 4 weeks and then moved to 2 to 8°C
for 12 weeks. Samples will be pulled for testing
every 2 weeks throughout protocol.
Lot Number Drug Product Stability Study Type Storage Condition Stability Data Data Table Location
Batch Use Start Date Presented
EL8723 Stability, Clinical, January 2021 Thermal Stress 25 +2 °C/60 +5% RH Table 3.2.P.8.3-2
(Pfizer, Puurs) Emergency Supply”,
Process performance 30+2°C/65 +5% RH Table 3.2.P.8.3-3
qualification (Complete)
(February 2021 | Thermal Cycling 1: Ultra frozen vials are placed at 16 weeks Table 3.2.P.8.3-19
for Thermal -20 #5 °C for 4 weeks and then moved to 2 to 8°C (On-going)
Cycling Study 1 | for 12 weeks. Samples will be pulled for testing
and April 2021 | every 2 weeks throughout protocol.
for Thermal Thermal Cycling 2: Ultra frozen vials are placed at 1 month Table 3.2.P.8.3-24
Cycling Studies | 394 5 °C for 4 weeks (1 month) and then moved
2&3) to -90 to -60 °C for the shelf life of the drug
product.
Thermal Cycling 3: Ultra frozen vials are placed at 1 month Table 3.2.P.8.3-25
5+3 °C for 4 weeks (1 month) and then moved to
-90 to -60 °C for the shelf life of the drug
product.
Ultrafrozen vials were cycled between 25 +2 °C/60 | 10 freeze thaw cycles Table 3.2.P.8.3-26
+ 5%RH (for 2 to 7 hours) and -90 to -60 °C (for 24
+2 hours) for a total of 10 cycles
EL3248 Stability, Clinical, December 2020 | Thermal Stress 25 +2 °C/60 +5% RH Table 3.2.P.8.3-11
(Pfizer, Kalamazoo) | Emergency Supply”, Thermal Stress 30+2 °C/65 +5% RH Table 3.2.P.8.3-12
Process performance
qualification
ET0384 Stability, Emergency | February 2021 | Thermal Cycling: Ultra frozen vials are placed at - Release Table 3.2.P.8.3-27
(Polymun/Pfizer, Supply”, Process 90 to -60 °C for 5 months and then a subset of vials
Puurs) performance moved to 2 to 8°C for 1 month. Samples pulled at
qualification 6M for testing at both -90 to -60°C and 2 to 8°C.
This is repeated at 11/12M, 17/18M and 23/24M
EL3249 Stability, Clinical, January 2021 Thermal Cycling: 1 week at -90 to -60°C , followed 8 weeks Table 3.2.P.8.3-23
(Pfizer, Kalamazoo) | Emergency Supply”, by 2 weeksat -20 +5 °C, 4 weeks at2 to 8°C and 1 (Complete)
Process performance week at 25 +2 °C/60 +5% RH.
qualification
EL9266 Stability, Emergency | February 2021 | Thermal Cycling: Ultra frozen vials are placed at - 16 weeks Table 3.2.P.8.3-22
CONFIDENTIAL
Page 5
090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT) BNT162b2 3.2.P.8.3. Stability Data Thermal — Stress and Cycling Table 3.2.P.8.3-1. Summary of Drug Product Thermal Stability Studies Page 6 Lot Number Drug Product Stability Study Type Storage Condition Stability Data Data Table Location Batch Use Start Date Presented EN1195 Stability, Emergency | February 2021 | Thermal Cycling: Ultra frozen vials are placed at - 12 weeks Table 3.2.P.8.3-18 (mibe/Pfizer, Puurs) | Supply“, Process 20 +5 °C for 4 weeks and then moved to 2 to 8°C (On-going) performance for 12 weeks. Samples will be pulled for testing qualification every 2 weeks throughout protocol. EK4242 Stability, Emergency January 2021 Thermal Cycling: 1 week at -90 to -60°C followed 5 weeks Table 3.2.P.8.3-20 (mibe/Pfizer, Puurs) | Supply“, Process by 4 weeks at 2 to 8°C (Complete) performance qualification EL7834 Stability, Emergency January 2021 Thermal Cycling: 1 week at -90 to -60°C followed 10 weeks Table 3.2.P.8.3-21 (Polymun Supply*, Process by 4 weeks at -20 +5 °C and then 4 weeks at 2 to (Complete) Scientific/Pfizer, performance 8°C. and 1 week at 25 +2 °C/60 +5% RH. Puurs qualification EH9899 Stability, November Thermal Stress 25+42°C/60+4+5%RH 1 month Table 3.2.P.8.3-13 (Pfizer, Kalamazoo) | Emergency Supply? 2020 (Complete) EJ1688 Stability, Emergency | November 2020 Thermal Stress 25 +2 °C/60 +5% RH 1 month (Complete) Table 3.2.P.3.3-4 (mibe/Pfizer, Puurs) | Supply? EK1768 Stability, Emergency | November 2020 Thermal Stress 25 +2 °C/60 +5% RH 1 month (Complete) Table 3.2.P.8.3-5 (Polymun Supply”, Clinical Thermal Cycling: 2 weeks at -90 to -60°C followed 14 weeks (Complete) Table 3.2.P.8.3-16 Scientific/Pfizer, inventory by 4 weeks at -20 +5 °C and then 8 weeks at2 to Puurs 8°C. Samples will be pulled for testing every 2 weeks throughout protocol. EJ1686 Stability, Emergency | November 2020 Thermal Stress 25 +2 °C/60 +5% RH 1 month (Complete) Table 3.2.P.8.3-6 (Polymun Supply*, Clinical Thermal Cycling: 2 weeks at -90 to -60°C followed 14 weeks (Complete) Table 3.2.P.8.3-17 Scientific/Pfizer, inventory by 4 weeks at -20+5 °C and then 8 weeks at2 to Puurs 8°C. Samples will be pulled for testing every 2 weeks throughout protocol. EJ1685 Stability, Emergency | November 2020 Thermal Stress 25 +2 °C/60 +5% RH 1 month (Complete) Table 3.2.P.8.3-7 (Polymun Supply®, Clinical Scientific/Pfizer, inventory Puurs EJ0553 Stability, Emergency | November 2020 Thermal Stress 25 +2 °C/60 +5% RH l month Table 3.2.P.8.3-8 (Polymun Supply®, Clinical (Complete) Scientific/Pfizer, inventory Puurs CONFIDENTIAL
090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT) BNT162b2 3.2.P.8.3. Stability Data Thermal — Stress and Cycling Table 3.2.P.8.3-1. Summary of Drug Product Thermal Stability Studies Lot Number Drug Product Batch Use Stability Start Date Study Type Storage Condition Stability Data Presented Data Table Location EE8493 Stability, Emergency | September 2020 Thermal Stress 25 +2 °C/60 +5% RH Table 3.2.P.8.3-9 (Polymun Supply®, Clinical Scientific/Pfizer, inventory Thermal Stress 30 +2 °C/65 +5% RH Table 3.2.P.8.3-10 Puurs) (Complete) BCV40420-A Stability, Clinical May 2020 Thermal Stress 25+2°C 4 months Table 3.2.P.8.3-14 (Polymun Scientific) (complete) Supportive Lots (BNT162b1) BCV10320-A Supportive Stability, April 2020 Thermal Stress 25+2°C 3 months Table 3.2.P.8.3-15 (Polymun Scientific) | Clinical (complete) a. Emergency supply designation applies to US market. b. A minimum of one PPQ lot will be enrolled in thermal stress and thermal cycling stability programs compliant with ICH Guidelines and further information on lot numbers, manufacture, stability enrollment and available data will be provided in the future. TBD = To Be Determined CONFIDENTIAL Page 7
090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT)
BNTI162b2
3.2.P.8.3. Stability Data
Thermal — Stress and Cycling
Table 3.2.P.8.3-2. Stability Data for Drug Product PPQ Lot EL8723 Stored at 25 + 2 °C/60 + 5% RH (Pfizer, Puurs)
Analytical Appearance pH Dynamic Light Scattering (DLS) Fluorescence Assay
ttribute ; , .
Polydispersity |Encapsulation
Timepoint / Acceptance | White to off-white suspension May contain white to
Criteria? off-white opaque,
amorphous particles
0 WOS Meets (EFVP)
1W WOS Meets (EFVP)
2W WOS Meets (EFVP)
IM WOS Meets (EFVP)
Analytical HPLC-CAD Cell-based Flow Cytometry Capillary Gel
Procedure/Quality Electrophoresis
Attribute i i A Integri
Timepoint / Acceptance
Criteria?
a. Acceptance criteria in place at time of testing.
W = Week, M = Month, S = To be Scheduled, NS = Not Scheduled at Time Point; EFVP = Essentially free from visible particulates; LNP = Lipid Nanoparticle; HPLC-CAD
= high performance liquid chromatography-charged aerosol detector, WOS = White to off-white suspension
CONFIDENTIAL
Page 8
090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT) BNT162b2 3.2.P.8.3. Stability Data Thermal — Stress and Cycling Table 3.2.P.8.3-3. Stability Data for Drug Product PPQ Lot EL8723 Stored at 30 £# 2 °C/65 + 5% RH (Pfizer, Puurs) Analytical Appearance | pH | Dynamic Light Scattering (DLS) Fluorescence Assay Procedure Quality Appearance (Visual) Visible Particulates LNP Size LNP RNA RNA Content ute . F . Polydispersity | Encapsulation Timepoint / Acceptance | White to off-white suspension May contain white to Criteria? off-white opaque, amorphous particles 0 WOS Meets (EFVP) 1W WwOS Meets (EFVP) 2W WOS Meets (EFVP) IM WOSs Meets (EFVP) Analytical HPLC-CAD Cell-based Flow Cytometry Capillary Gel Procedure/Quality Electrophoresis Attribute ALC-0315 Content ALC-0159 Content DSPC Content Cholesterol Content In Vitro Expression RNA Integri Timepoint / Acceptance Criteria? 0 1W 2W 1M a. Acceptance criteria in place at time of testing. Updated at the 1 month time point. W = Week, M = Month, S = To be Scheduled, NS = Not Scheduled at Time Point; EFVP = Essentially free from visible particulates; LNP = Lipid Nanoparticle; HPLC-CAD = high performance liquid chromatography-charged aerosol detector, WOS = White to off-white suspension CONFIDENTIAL Page 9
090177e1972448e5\Approved\Approved On: 26-Jul-2021 03:10 (GMT)
BNTI162b2
3.2.P.8.3. Stability Data
Thermal — Stress and Cycling
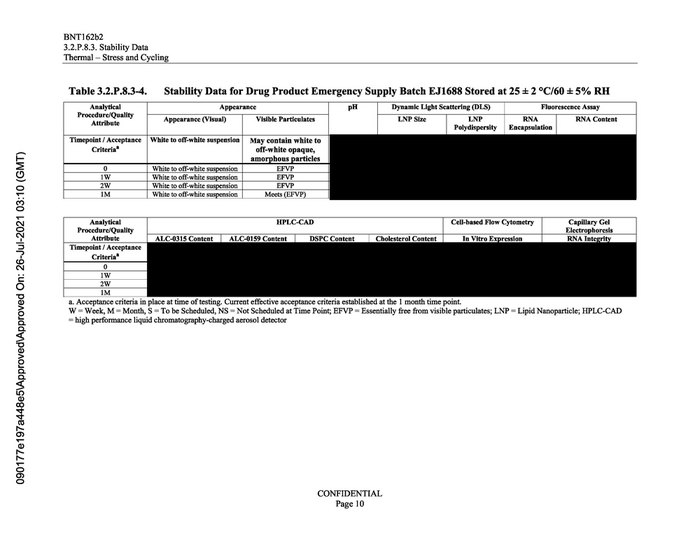
Table 3.2.P.8.3-4.
Stability Data for Drug Product Emergency Supply Batch EJ1688 Stored at 25 £# 2 °C/60 #5% RH
White to off-white suspension
EFVP
Fluorescence Assay
Analytical Appearance pH | Dynamic Light Scattering (DLS)
Procedure/Quality Appearance (Visual) Visible Particulates LNP Size LNP
Attribute . .
Polydispersity
Timepoint / Acceptance | White to off-white suspension | May contain white to
Criteria? off-white opaque,
RNA
Encapsulation
RNA Content
Timepoint / Acceptance
Criteria?
a. Acceptance criteria in place at time of testing. Current effective acceptance criteria established at the 1 month time point.
W = Week, M = Month, S = To be Scheduled, NS = Not Scheduled at Time Point; EFVP = Essentially free from visible particulates; LNP = Lipid Nanoparticle; HPLC-CAD
= high performance liquid chromatography-charged aerosol detector
CONFIDENTIAL
Page 10
1W White to off-white suspension | EFVP
2W White to off-white suspension | EFVP
1M White to off-white suspension | Meets (EFVP)
Analytical HPLC-CAD Cell-based Flow Cytometry Capillary Gel
Procedure/Quality Electrophoresis
Attribute ALC-0315 Content ALC-0159 Content DSPC Content Cholesterol Content RNA Integrii