R - bendamustine hydrochloride-PSUSA-3162-202001-PRAC AR_BfArM
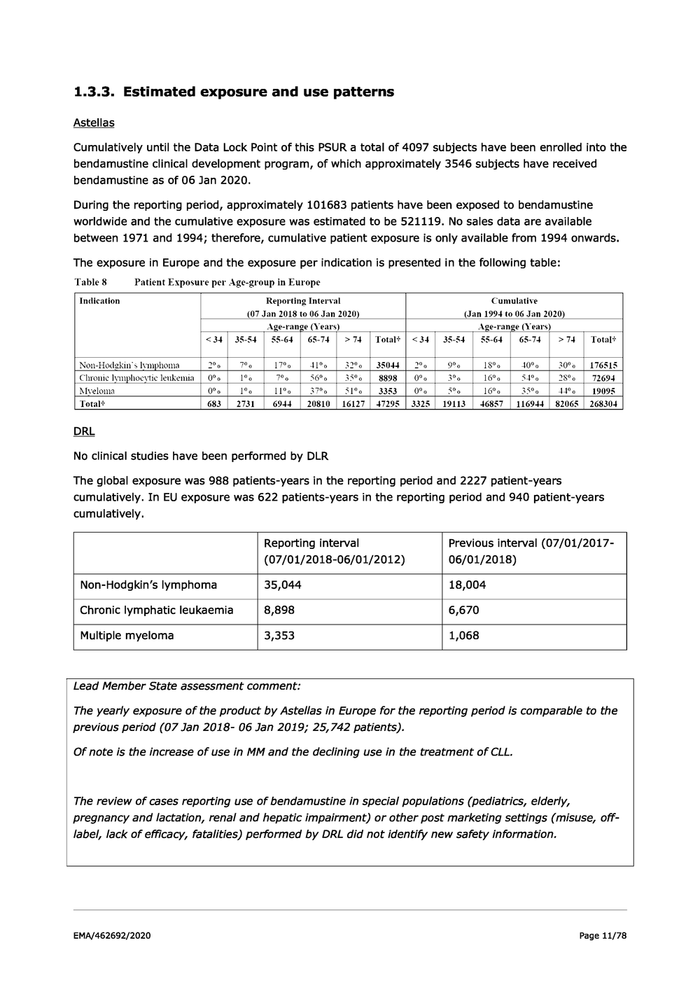
1.3.3. Estimated exposure and use patterns Astellas Cumulatively until the Data Lock Point of this PSUR a total of 4097 subjects have been enrolled into the bendamustine clinical development program, of which approximately 3546 subjects have received bendamustine as of 06 Jan 2020. During the reporting period, approximately 101683 patients have been exposed to bendamustine worldwide and the cumulative exposure was estimated to be 521119. No sales data are available between 1971 and 1994; therefore, cumulative patient exposure is only available from 1994 onwards. The exposure in Europe and the exposure per indication is presented in the following table: Table 8 Patient Exposure per Age-group in Europe Indication Reporting Interval Cumulative (07 Jan 2018 to 06 Jan 2020) (Jan 1994 to 06 Jan 2020) Age-range (Years) Age-range (Years) <34 | 35-54 | 55-64 | 65-74 | >74 | Total# | <34 | 35-54 | 55-64 | 65-74 >74 | Total’ Non-Hodekin’s Iyrmphoma 2°0 700 17°0 41% 32% | 3504 | 2% 9°%0 18° 40° 30° | 176515 Chronic Iymphoeytic leukemia_| 0° 1° 700 56° 350 | 8898 0°0 3°%0 16°0 54° 28% | 72694 Myeloma 0° 1° 11°o 37% 510 | 3353 0°0 So 16°0 35° 44% 19095 Total* 683 | 2731 6944 | 20810 | 16127 | 47295 | 3325 | 19113 | 46857 | 116944 | 82065 | 268304 DRL No clinical studies have been performed by DLR The global exposure was 988 patients-years in the reporting period and 2227 patient-years cumulatively. In EU exposure was 622 patients-years in the reporting period and 940 patient-years cumulatively. Reporting interval Previous interval (07/01/2017- (07/01/2018-06/01/2012) 06/01/2018) Non-Hodgkin’s Iymphoma 35,044 18,004 Chronic Iymphatic leukaemia 8,898 6,670 Multiple myeloma 3,353 1,068 Lead Member State assessment comment: The yearly exposure of the product by Astellas in Europe for the reporting period is comparable to the previous period (07 Jan 2018- 06 Jan 2019; 25,742 patients). Of note is the increase of use in MM and the declining use in the treatment of CLL. The review of cases reporting use of bendamustine in special populations (pediatrics, elderly, pregnancy and lactation, renal and hepatic impairment) or other post marketing settings (misuse, off- label, lack of efficacy, fatalities) performed by DRL did not identify new safety information. EMA/462692/2020 Page 11/78
1.3.4. Data in summary tabulations The MAHSs provided summary tabulations with the global number of cases and cases by SOC, sorted by seriousness. MedDRA version 22.1 was used for coding. Astellas The summary tabulations include 3114 SAEs (cumulatively) from clinical trials and 26,505 ADRs (cumulatively) from post-marketing data sources related to bendamustine. In the reporting period 5856 ADRs were reported from post-marketing data sources. 5294 ADRs resulted from spontaneous sources and literature and out of these 2305 ADRs were classified as serious. Cumulatively, a total of 26505 ADRs have been received from post-marketing data sources. DRL No clinical studies have been performed. During the reporting interval, a total of 782 adverse events with 640 serious reactions/events and 142 non-serious reactions events were reported. During the cumulative period, from DRL/betapharm/Altan IBD, a total of 933 adverse events of which 777 serious reactions/events and 156 non-serious reactions events were reported. Lead Member State assessment comment: The MAHs have presented a cumulative summary tabulation of serious adverse events from clinical trials and a cumulative and interval summary tabulation of adverse reactions from post-marketing data ADRs from Post Marketing Experience. Overall, the data in the summary tabulations do not raise any new safety concern. 1.3.5. Findings from clinical trials and other sources Astellas Completed clinical trials There were no completed interventional clinical trials during the reporting interval for bendamustine. Ongoing clinical trials During the reporting period, 3 Licence Partner-sponsored interventional clinical trials were ongoing. End-of-Text Table 1 Overview of Ongoing Clinical Trials Study ID and Sponsor Study Title Phase 3 A randomized. double-blind. placebo-controlled phase 3 study of BTK inhibitor. PCI-32763 (ibrutinib). in combination with either BR or R-CHOP in subjects with previously treated imdolent NHL A randomuized. double-blind. placebo-controlled phase 3 studv PCI-32765MCL3002 (Janssen) | ofthe BTR Inhibitor. PCI-32765 (ibnutinib). in combination with BR in subjects with newly diagnosed MCL A multi-center. open-label phase 3 study of SyB L-0501 in 2017002 (SynıBio) combination with riruximab in patients with recument or telapsed diffuse large B-cell Iymphoma BR: Bendamustine and Rituximab: BTK: Bruton’s Tyresine Kinase: MCL: Mantle Cell Lymphoma: NHL: Non-Hodgekin’s Lymphoma: R-CHOP: Riruximab. Cyclophosphanude. Doxorubicin. Vinenstine. and Prednisone PCI-32765FLR3001 (Janssen) EMA/462692/2020 Page 12/78

Overall, no new safety concern or information with potential impact on the benefit-risk profile of bendamustine was reported from these ongoing clinical trials. Long-term follow-up During this reporting period, 1 Licence Partner-sponsored long-term follow-up study was completed (BDM3502 sponsored by Mundipharma) and 1 Licence Partner-sponsored long-term follow-up study was ongoing (PCI-32765CLL3001 sponsored by Janssen). Additionally, 1 study (Study 3076, sponsored by Teva) was included as ongoing in Section 7.3 in the previous bendamustine PSUR (07 Jan 2017 - 06 Jan 2018). This study was completed and the final addendum to the clinical study report (CSR) was signed-off on 17 Oct 2017. It was an open label study to evaluate bendamustine in the treatment of Chinese patients with indolent NHL refractory to rituximab treatment. Patients who completed or were withdrawn from study drug treatment and did not exhibit disease progression entered the follow-up period for up to 2 years and were monitored for disease progression, initiation of another treatment for the disease or death. The results of this study showed bendamustine to be an effective treatment for patients with rituximab-refractory, relapsed, indolent NHL, with an overall response rate (ORR) of 73%. In view of the relatively higher incidence of respiratory failure, pneumonia, lung infection, and herpes virus infection in this study compared with current standards of care and that the incidence may not be explained by bone marrow suppression alone, physicians should pay particular attention to the risk of lung injury and immunosuppression. The efficacy and safety results for the 2-year long-term follow-up period did not alter the conclusions of the initial report. Study BDM3502 (Sponsor Mundipharma) This study was a multicenter, phase 3, randomized, open-label, 2-arm, parallel-group study to test the efficacy of bendamustine in the treatment of subjects with indolent B-cell NHL refractory to rituximab. The aim of the study was to investigate the efficacy of bendamustine compared to treatment of physician’s choice (TPC) in the treatment of subjects with indolent NHL refractory to rituximab. Subjects were randomized to receive either bendamustine therapy or TPC using a 2:1 randomization scheme. Subjects were assigned to study treatment in accordance with the approved randomization schedule for the study. It was planned to randomize approximately 125 subjects into the study; a total of 109 subjects were enrolled. In total, 88 subjects were randomized and treated and the total number of events was 41, which was less than half of the needed subjects number required for a final analysis. The primary endpoint was not achieved due to low subjects number, as a result of early termination, and consequently low statistical power. Although a numerical difference of more than 7 months in progression-free survival (PFS) and a numerical difference of more than 20% in ORR and more than 7 months longer duration of response all favoring the bendamustine arm could be seen, these results did not reach statistical significance. Of note is that disease progression in subjects was delayed longer than hypothesized (5 months and 9 months in the TPC and bendamustine groups, respectively) and subjects’ median survival time were longer than the hypothesized study duration (estimated maximum study duration of 31.2 months). The study was not designed to be able to distinguish safety versus a standardized comparator treatment as the bendamustine treatment regimen was more fixed, and the study was open-label. Thus, the numerically higher reporting rates, driven by a pattern of known events associated with bendamustine treatment, should not be over-interpreted, also recognizing that 40 (69%) of bendamustine subjects completed 6 cycles of treatment compared to 8 (29.6%) of TPC subjects, and 16 (27.6%) of bendamustine compared to 1 (3.7%) of TPC subjects continued through 8 cycles of EMA/462692/2020 Page 13/78

treatment. The number of reports, in particular the limited number of SAEs, and of suspected unexpected serious adverse reactions, was well within what could be expected in a subject population with prior history of chemotherapy. The safety analysis of bendamustine did not reveal any new safety signals and remained within the expected safety profile known for this substance. This supports the conclusion that the known safety profile of bendamustine is confirmed by the results of this study. Lead Member State assessment comment: Study BDM3502 did not identify any new significant safety information. The efficacy data of this study is assessed within the ongoing Renewal procedure. Study PCI-32765CLL3001 (Sponsor Janssen) Study PCI-32765CLL3001 is a randomized (1:1), double-blind, placebo-controlled, international, multicenter, phase 3 study that evaluates the benefits and risks of combining ibrutinib, and bendamustine and rituximab (BR) in subjects with relapsed or refractory CLL/small Iymphocytic Iymphoma (SLL) following at least 1 line of prior systemic therapy. Overall, no new safety concern or information with potential impact on the benefit-risk profile of bendamustine was reported from this ongoing long-term follow-up study. Lead Member State assessment comment: Study PCI-32765CLL3001 did not identify any new significant safety information. Other therapeutic use Bendamustine is not used for any therapeutic indication other than those described in Section 1. During the reporting period, 1 expanded access program was ongoing in Iraq and 1 expanded access program was completed in Tunisia. No new clinically important safety information was identified from these programs. Fixed combination therapies Bendamustine in combination with other therapies is being evaluated in 4 ongoing clinical trials: e Study PCI-32765MCL3002 (sponsored by Janssen): It is evaluating bendamustine in combination with ibrutinib and rituximab in patients newly diagnosed with MCL. e Study PCI-32765FLR3001 (sponsored by Janssen): It is evaluating bendamustine in combination with ibrutinib and rituximab in patients previously treated for indolent NHL. e Study PCI-32765CLL3001 (sponsored by Janssen): It is evaluating bendamustine in combination with ibrutinib and rituximab in patients with relapsed or refractory CLL/SLL. e Study 2017002 (sponsored by SymBio): It is evaluating bendamustine in combination with rituximab in patients with recurrent or relapsed diffuse large B-cell Iymphoma (DLBCL). Overall, no new safety concern or information with potential impact on the benefit-risk profile of bendamustine was reported from these clinical trials. Non-interventional studies EMA/462692/2020 Page 14/78

Table 11 Overview of Non-interventional Studies
Ongoing Non-interventional Stndies
Study ID and Sponsor Trial Description
BO0501-M065-501 Post-marketing surveillance of Symbenda injection (bendamustine
(Eisai. a LP of SymBio) hydrochloride) in Korean patients
TRA03S The drug-use inyestigation for chronice I\ymphocytic leukemia of
{SymBio} Treakisym injection
PMS-PL-LEV-2012-01 Cha racteristic sof population and nen parame ter 5 for treatinent
(Mundipharma) conduct in CLL patients prescribed bendanwstine as first line
treatment
RRA-20448 To collect adverse events during the first 2 years marketing
{Janssen) throush surveys in Mexico
231-M A-3264 Non-interventional post-authorization safety study (NI-PASS) as
{Astellas} an effectiveness check of an additional risk minimization measure
{aRMM) (direct healthcare professional conmmunmication [DHPC])
for bendamustine
Completed Non-interventional Study
Study ID and Sponsor Trial Description
BDM9501 The efficacy and safety of Levact treatment for patients witlı CLL.
{Mundipharma) NHL and MM
CLL: Chronic Lyinphoeytic Leukemia. LP: License Partner. MM: Multiple Myeloma. NHL: Non-Hodskin’s
Lymphoma
There is no relevant safety information that became available from non-interventional studies for
bendamustine during the reporting period.
Other clinical trial/study sources
During the reporting period, 17 investigator-initiated trials (IITs) have been conducted, of which 9
were supported by Mundipharma, 7 by Teva and 1 by Eisai, a LP of SymBio. No significant safety
findings were identified from these ongoing IITs.
Lead Member State assessment comment:
No new significant safety information was identified from combination therapies, non-interventional
studies or other study sources.
Non-clinical data
No nonclinical studies were ongoing or completed during the reporting period.
Literature
A review of the published scientific literature for bendamustine was conducted for the reporting
interval. A literature search was executed in Embase, Medline and Biosis for bendamustine for the
period of 07 Jan 2018 to 06 Jan 2020. Three publications addressed bendamustine and hepatitis E
infections during the reporting period (refer to Section 15.3.2).
Four publications addressed information from GALLIUM and GADOLIN studies.
GALLIUM and GADOLIN Trials
Gibiansky E, Gibiansky L, Buchheit V, Frey N, Brewster M, Fingerle-Rowson G, et al. Pharmacokinetics,
exposure, efficacy and safety of obinutuzumab in rituximab-refractory follicular Iymphoma patients in
the GADOLIN phase III study. Br J Clin Pharmacol. 2019;85(9):1935-45.
EMA/462692/2020 Page 15/78

This publication aimed to further characterize the pharmacokinetics of obinutuzumab in different CD20 B-cell malignancies and to explore whether differences in drug exposure in rituximab- relapsed/refractory follicular Iymphoma (FL) patients from the GADOLIN study (a phase 3 trial to assess the efficacy, safety and pharmacokinetics of obinutuzumab and bendamustine [G-Benda] versus bendamustine monotherapy in rituximab-refractory indolent NHL) affected pharmacodynamics, safety or efficacy. Safety outcomes explored were occurrence of SAEs, severity of infusion-related reactions, occurrence, and severity of neutropenia and thrombocytopenia AEs, and change in neutrophil/platelet counts. The Cox proportional hazards models included 294 patients for PFS (obinutuzumab [G-Benda], n = 128; control [bendamustine-only], n = 166) and 295 for OS (129 and 166, respectively). The PFS analysis with continuous exposure confirmed that the risk of a PFS event was lower in the G- Benda arm than the bendamustine-only arm. Longer PFS was observed in all obinutuzumab groups, compared to the bendamustine-only group. Graphical analysis revealed no relationships between exposure and AEs, and changes in neutrophil and platelet counts during study treatment were unaffected by the level of obinutuzumab exposure. However, in multivariate Cox regression analysis, the selected obinutuzumab dosing regimen offered clinical benefit in a majority of rituximab-refractory FL patients treated with bendamustine, irrespective of variability in exposure while minimizing AEs. MAH Comments: Obinutuzumab is approved for previously untreated CLL, and for use with bendamustine in patients with rituximab-relapsed/refractory FL. In rituximab-relapsed/refractory FL patients in the GADOLIN study, no association was found between G-Benda exposure and AEs, confirming the favorable safety profile of bendamustine combined with obinutuzumab in these patients. Jamois C, Gibiansky E, Gibiansky L, Buchheit V, Sahin D, Cartron G, et al. Role of obinutuzumab exposure on clinical outcome of follicular Iymphoma treated with first-line immunochemotherapy. Br J Clin Pharmacol. 2019;85(7):1495-1506. This publication studied the sources of variability in outcome amongst front line FL obinutuzumab- treated patients in the GALLIUM trial with particular emphasis on whether differences in obinutuzumab exposure affected outcome, specifically if lower obinutuzumab exposure affected clinical outcome. Despite a higher cumulative dose during induction (3-weekly regimen for 6-8 cycles) and generally higher obinutuzumab exposure in obinutuzumab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (G-CHOP)/ Cyclophosphamide, Vincristine, and Prednisone (CVP) compared to G-Benda patients (4-weekly regimen for 6 cycles), it was found that lower exposure was associated with shorter PFS in patients treated with G-CHOP or obinutuzumab + CVP, but that PFS was similar across the range of obinutuzumab exposure in patients treated with G-Benda. The faster decrease of time- dependent obinutuzumab clearance in G-Benda compared to G-CHOP/CVP patients (half-life of decrease of 13.2 versus 21.6 days), elucidated by the pharmacokinetic analysis suggests a faster reduction of CD20+ target by G-Benda treatment. Exposure to obinutuzumab is influenced by several factors such as patient demographics, tumor burden and chemotherapy backbone (during the first few weeks of treatment) and is therefore variable among patients. Nevertheless, this variability in exposure did not correlate with clinical outcome in patients treated with G-Benda. In those patients, intrinsic patient characteristics (male sex and tumor burden) are relevant contributors to the differences in PFS. MAH Comments: Similar PFS across the range of obinutuzumab exposure in patients treated with G- Benda compared to shorter PFS associated with lower exposure in patients treated with G-CHOP or obinutuzumab + CVP supports the fact that bendamustine is often considered to be more effective than cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) and CVP for FL. There was no specific bendamustine safety information retrieved from this article. EMA/462692/2020 Page 16/78

Pott C, Sehn LH, Belada D, Gribben J, Hoster E, Kahl B, et al. MRD response in relapsed/refractory FL after obinutuzumab plus bendamustine or bendamustine alone in the GADOLIN trial. Leukemia. 2020;34(2):522-532. DOI: 10.1038/5s41375-019-0559- 9. Epub 2019 Aug 28. This publication reported the results of a preplanned minimal residual disease (MRD) assessment in patients with FL enrolled in GADOLIN. The main objectives of the analysis were: (1) to evaluate the depth and time course of the MRD response to G-Benda or bendamustine by assessing MRD during induction (mid-induction) and at end of induction (EOI), and throughout obinutuzumab maintenance (G-Benda arm) or follow-up (bendamustine arm); and (2) to assess the association between EOI MRD status and PFS and OS using updated efficacy data with a median follow-up of 31.8 months. At mid- induction, 41/52 (79%) patients receiving G-Benda were MRD-negative versus 17/36 (47%) patients receiving bendamustine alone (P = 0.0029). At EOI, 54/63 (86%) patients receiving G-Benda were MRD-negative versus 30/55 (55%) receiving bendamustine alone (P = 0.0002). MRD-negative patients at EOI had improved PFS (hazard ratio [HR], 0.33, 95% Confidence Interval [CI], 0.19-0.56, p < 0.0001) and OS (HR, 0.39, 95% CI, 0.19-0.78, P = 0.008) versus MRD-positive patients, and maintained their MRD-negative status for longer if they received obinutuzumab maintenance than if they did not. MAH Comments: This article was heavily focused on the efficacy of G-Benda when compared to bendamustine alone for FL. The results suggest that the addition of obinutuzumab to bendamustine based treatment during induction can significantly contribute to the speed and depth of response, and obinutuzumab maintenance in MRD-negative patients potentially delays Iymphoma regrowth. There was no specific bendamustine safety information retrieved from this article. Watts EMR, Osborne WL, Elamain A, Lennard A. Is rituximab maintenance really associated with as much mortality as seen in gallium? Real world data from the North East of England. Br J Haematol. 2018;181:86-7. This publication compares the outcomes of the GALLIUM study (study to directly compare obinutuzumab-chemotherapy and maintenance and rituximab-chemotherapy maintenance in patients with FL) with a cohort of patients (n = 110) in North-East of England with Iymphoma who had received rituximab-chemotherapy and had rituximab maintenance from 01 Jul 2012 to 30 Sep 2017. In GALLIUM, bendamustine was associated with higher rates of severe infection, and a non-relapse fatal event rate of 5.6% in the obinutuzumab arm and 4.4% in the rituximab arm. In the cohort on 110 patients in North-East of England, data were collected on infection rates and severity, neutropenia, non-hematological toxicity and mortality. The 46% of this cohort had FL, with high grade Iymphoma/background FL as the next largest subgroup. Thirty-six percent received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), 27% BR, 16% rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP) and 3.6% received a combination of R- CVP/CHOP. Small numbers of patients received other rituximab-chemotherapy regimens. Forty percent of patients in R-CHOP cohort (n = 40) had infection during their maintenance period, although these were all grade 1-2. The 36.6% of patients who received BR (n = 30) had infection and these tended to be more severe and recurrent, compared with 23% of the cohort suffering grade 3-5 infection. There were no fatal infections across the whole cohort of 110 patients. Of the 2 deaths in the bendamustine cohort, 1 was due to relapsed Merkel cell carcinoma (MCC) and 1 was due to Parkinson’s disease and progressive FL. The 5.45% (6 out 110) of the patients in this cohort died. One of the deaths was attributed to treatment toxicity (anthracycline cardiac toxicity). The increase in grade 3-5 infections in patients receiving a bendamustine chemotherapy backbone in combination with rituximab maintenance was concordant with data from the GALLIUM study. The outcome of the cohort data, however did not correspond to the 5% infective mortality rate seen with bendamustine in the GALLIUM study. EMA/462692/2020 Page 17/78

MAH Comments: The analyzed data of the cohort of 110 patients have not revealed the 5% infective mortality rate seen with bendamustine in the GALLIUM study. The rate of infections in GALLIUM study in R-CHOP arm (all grade 1-2), and in BR arm was within similar range (40% versus 36.6% respectively). An increased rate of severe infections (grade 3-5) in the bendamustine arm (23%) was recognized with no fatal outcomes. Serious infections are known for bendamustine and are in the RSI of this product. DRL A review of the literature, covering the interval reporting interval, has been conducted in PubMed for safety studies and other potentially safety relevant reports associated with bendamustine use. The literature search revealed no relevant safety literature studies. Lead Member State assessment comment: The MAH Astellas presented four literature reports with results from the Gadolin and Gallium trials. No new significant safety information was identified. Literature concerning targeted medical events is presented and discussed in the respective sections. Other periodic report During the reporting interval, 1 bendamustine Periodic Benefit-risk Evaluation Report covering the period from 17 Dec 2018 to 16 Dec 2019 was prepared by Astellas. There were no significant findings identified from this report. Medication errors The MAH identified 40 cases within SMQ (narrow) medication errors reporting a total of 105 events, of which 41 events were classified as a medication error. Out of these 40 cases, there were 24 cases that reported a medication error without any additional AEs, 14 medication error cases that reported additional AE, and 2 cases of intercepted errors. Of the 14 cases that reported an additional event, 8 cases were assessed as serious. The most common reported PT is PT Incorrect dose administered (n=10). Special situations No new safety information was identified from the review of the following special situations for bendamustine: «e Eliderly e Lack of effect e Mecdication error e _Occupational exposure «. Off-label use e _Overdose/Drug abuse/Misuse « Pediatric e Pregnancy/Breastfeeding EMA/462692/2020 Page 18/78

Off-label use in paediatric patients has been reported Feb 2018 in Egypt without associated events reported. Lead Member State assessment comment: No new significant safety information associated with medication errors or special situations was identified during the reporting period. DRL The MAH did not perform clinical studies, non-interventional studies, other studies or non-clinical studies during the reporting period. The MAH identified 8 medication errors. The most common medication error was prescribed under dose (n=4). The MAH reviewed literature covering the reporting interval. No relevant safety information was identified. Lead Member State assessment comment: No new safety information was identified by DRL. 1.3.6. Lack of efficacy in controlled clinical trials During the reporting period, no relevant information on lack of efficacy was identified that could reflect a significant risk to the clinical trial subjects. 1.3.7. Late-breaking information No potentially important safety, efficacy and effectiveness findings arose after the data lock point of the PSUR 2. Signal and risk evaluation 2.1. Summary of safety concerns Astellas The important safety concerns recognized for bendamustine at the beginning of the current reporting period are: EMA/462692/2020 Page 19/78

Table 20 Sunımary of Safety Concerns Important identified risks | « Miyelosuppression * Infections (including opportunistic infections ofherpes zoster. cytomegaloviris. Preimoevstis jFroveci pneumonia} » Hepatitis B re-activation * Hepatic failure » Severe skin reactions * Cardiac disorders of cardiac failure. myocardial infarction. and atrial fibrillation Tumour Isis syndrome Renal failure Anaplıylaxıs Secondary malignancies ofmyelodysplastic syndrome and acute myeloid leukemia Important potential risks |« None Alissing information « Exposure during pregnancy and lactation Source: FU Risk Management Plan for bendamustine. version 6. dated 02 Oct 2017. The risk definitions were adopted as per the new EU Risk Management Plan (RMP) guidance and the template which is based on EMA’s Good Pharmacovigilance Practices Module V Revision 2. The safety concerns at the end of the reporting period in the RMP are: Table 21 Summary of Safety Concerns at the End of the Reporting Interval Important identified risks | « Myelosuppression + Infections (inchuding opportunistic infections ofherpes zoster. cyromegalovirus. Preimmocvstis jfrovecti pneumonia) » Hepatitis B re-activation Important potential risks |« None Alissing information » None RL The important safety concerns recognized for bendamustine at the beginning of the current reporting period are: Table 3: Summary of Safety Concems kKhyeksuppression In’ezions Zevere skin reactions Cardiac disorders Turmeurlysis syndrome Zrug hypersensibwity Important Zecondanr malignangies petential isks lan Senalioxichy JTepati failure Missing =ajents below age 15 years information Zxpssure during pregnaney and station =ffer in diferen: races EMA/462692/2020 Page 20/78










