2.Bescheid.Anlage8_VB_BioNTech
Dieses Dokument ist Teil der Anfrage „Vereinbarungen mit den Firmen BioNTech SE, CureVac AG und IDT Biologika GmbH in Bezug auf Impfstoffe gegen SARS-CoV-2“
Project description: “Accelerated Development of mRNA-based BNT162 COVID-19 Vaccine” Table of content 1. Introduction .................................................................................................................................... 2 a. Brief description of the applying company/institution (0,5 page) ............................................. 2 b. Executive Summary in tabular form (1 page max.) .................................................................... 2 2. Objectives........................................................................................................................................ 3 a. Scientific and/or technical working objectives of the project.................................................... 3 i. Objectives of clinical development, including objectives for adjustments/ expansions of manufacturing, processing and filling capacities within the clinical trial process........................... 4 ii. Objectives regarding collaboration with development and production partners during clinical trials..................................................................................................................................... 5 iii. Further objectives after market approval e.g. targeted production capacity, cooperation with licensees and other partners (scale-up and scale-out) ........................................................... 5 3. State of the art; previous work ....................................................................................................... 6 a. Quality and development status of the preparatory work ........................................................ 6 i. Clinical trial ............................................................................................................................ 9 ii. Vaccine production .............................................................................................................. 12 iii. Declaration on intellectual property rights.......................................................................... 17 b. Previous work of the applicant ................................................................................................ 19 4. Detailed description of the work plan........................................................................................... 22 a. Milestone Schedule .................................................................................................................. 22 b. Plans to accelerate the development/expansion of clinical trial and production capacities... 22 i. Acceleration of development .............................................................................................. 22 ii. Expansion of clinical trial capacities..................................................................................... 24 iii. Expansion of production capacities ..................................................................................... 25 c. Risk assessment, mitigation and avoidance ............................................................................. 28 5. Exploitation and Dissemination Plan............................................................................................. 30 a. Brief overview .......................................................................................................................... 30 b. Aspects of economic, scientific and technical exploitation prior to marketing authorisation . 31 c. Aspects of exploitation after marketing authorisation ............................................................ 31 6. Division of labour/cooperation with third parties ........................................................................ 33 7. Financing of the project, cost estimates ....................................................................................... 34 Own contribution, BMBF contribution, third-party financing........................................................... 35 Annex I – Milestone Planning................................................................................................................ 37 Annex II – References............................................................................................................................ 38 1

Project description Note he project description must contain all statements required here t is possible to deviate rom this template but this might complicate the application process 1. Introduction a. Brief description of the applying companyj/institution (0,5 page) BioNTech SE (BNT or the company) is a German, next generation immunotherapy company pioneering novel therapies for cancer, infectious and other serious diseases. BIONTECH was founded in 2008 by Prof. Dr. Ugur Sahin, PD Dr. Özlem Türeci and Prof. Christoph Huber, MD, based on their ground-breaking research in immunology. The company executed an IPO at Nasdaq in October 2019 in order to ensure long-term financing. The company has 6 sites in Germany and 2 in the US, employing more than 1,400 employees in total — of which 80% work in either R&D or manufacturing. BioNTech exploits multiple technology platforms, and is clinically developing product candidates in four distinct drug classes, in pursuit of its goal to develop individualized immunotherapies for cancer as well as other diseases. BioNTech’s leading technology is with messenger RNA-based immunotherapies (mRNA). Since 2013, BioNTech has been studying mRNA in humans and holds one of the world’s most advanced understandings of the technology’s impact on the human body — crucial knowledge for developing effective vaccines. 9 mRNA-based product candidates have advanced into clinical studies. BioNTech has built a fully integrated company, operating three GMP-certified manufacturing facilities in Germany. The mRNA used in the clinical trials in Germany and the US are exclusively produced by BioNTech in Mainz and Idar-Oberstein. BioNTech intends to further invest in local manufacturing capacities as its ability to develop, control and optimize the manufacturing of product candidates is a core strategic pillar and competitive advantage, especially for the individualized mRNA product candidates. b. Executive Summary in tabular form (1 page max.) Applicant (companyj/institution, BioNTech SE, attn. Dr. Christian Miculka project manager) An der Goldgrube 12 55131 Mainz Germany T: +49 160 3884823 F: +49 6131 9084-390 Vaccine technology (max. 140 Three established messenger RNA (mRNA) technologies: characters) unmodified uridine RNA (uRNA), nucleoside-modified mRNA (modRNA,) and self-amplifying mRNA (saRNA). Target Antigen (max. 140 Candidates are targeting the spike protein (S-protein), either characters) encoding the whole protein, a mutated version (P2 S) or the receptor binding domain (RBD) as antigen. Status of preclinical development, Pre-clinical development for the approval of Phase 1 trials (max. 100 words) has been concluded, initial clinical trials have already started in Germany and in the US. Additional pre-clinical work is still being carried out for a potential second

generation of vaccines, and to further inform clinical development. BioNTech (BNT), in collaboration with Pfizer, aims to apply its innovative mRNA vaccine technology platforms, combined expertise and global manufacturing & distribution capacities to contribute to the fight of the ongoing SARS-CoV-2 pandemic by targeting the following main objectives: OBJECTIVE 1 - CLINICAL DEVELOPMENT Provide an efficacious mRNA-based COVID-19 vaccine (BNT162 vaccine) by the end of 2020. OBJECTIVE 2 - MANUFACTURING SCALE-UP Supply an initial 100M doses of the vaccine by the end of 2020 and, in parallel, scale up EU manufacturing facilities to enable supply of -1.2 billion vaccine doses in 2021.

i. Objectives of clinical development, including objectives for adjustments/ expansions of manufacturing, processing and filling capacities within the clinical trial process PROVIDE AN EFFICACIOUS mRNA-BASED COVID-19 VACCINE (BNT162 VACCINE) BY THE END OF 2020. This objective focusses on the rapid preclinical & clinical development of BNT162 vaccine candidates to hopefully enable conditional/accelerated approval by Oct 2020, and traditional regulatory approval by Dec 2020, by targeting the following sub-objectives: Conclude IND-/CTA-enabling preclinical work with several vaccine candidates from 3 different mRNA-technology platforms (BNT162 vaccine candidates) Pursue rapid approval of clinical phase 1/2 trials by regulatory agencies (PEI & FDA) in Germany and the US Describe the safety, tolerability and immunogenicity profiles of 4-5 prophylactic BNT162 vaccines in healthy adults after 1 or 2 doses (-650 subjects) Pursue regulatory advice and approval for a global, scalable phase 2/3 trial with a selected candidate and dosing regimen Describe efficacy profile of selected BNT162 vaccine in healthy adults (up to 30,000 subjects) Achieve accelerated approval or „emergency use authorization“ (US) and conditional approval (Germany) based on phase 2 data (-3,000 subjects) Achieve traditional approval based on combined phase 2/3 data (-30,000 subjects) SUPPLY AN INITIAL 100M DOSES OF THE VACCINE BY THE END OF 2020 AND, IN PARALLEL, SCALE UP MANUFACTURING FACILITIES TO ENABLE SUPPLY OF -1.2 BILLION VACCINE DOSES IN 2021 This objective focusses on the accelerated expansion of manufacturing capacities, in parallel to (pre)clinical development and carried out fully at risk, by targeting the following sub-objectives: Provide pandemic supply of vaccine in November / December 2020 (100 million doses) 4
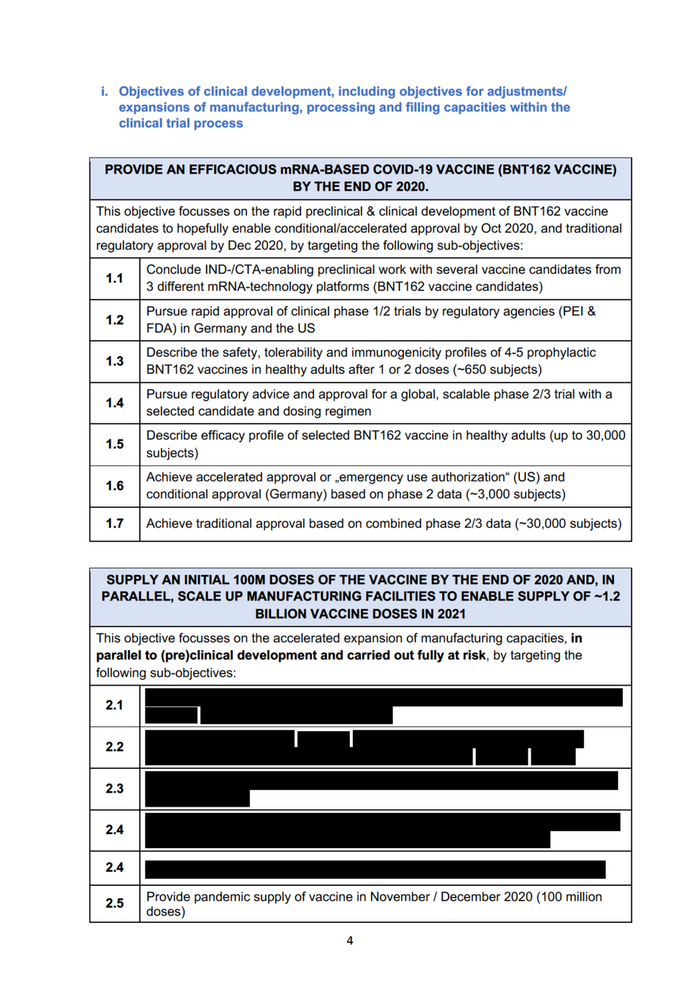
FT P—m Provide world-wide supply of vaccine in 2021 (1.2 billion doses produced in European and worldwide plants) ii. Objectives regarding collaboration with development and production partners during clinical trials BioNTech has concluded a collaboration agreement with Pfizer in March 2020, which is based on a 50:50 cost sharing model and covers the full clinical development and production of a BioNTech vaccine against SARS-CoV-2. BioNTech 's main reason for collaborating with Pfizer for clinical development and production was to access and leverage Pfizer's experience and manufacturing capacity to ensure clinical development on a global scale, while BioNTech retains sponsorship of the trials and its own manufacturing units and contractors in Germany and the EU. Additionally, a partnership with Fosun Pharma was concluded to jointly conduct clinical trials in China and to further support global supply where it is most needed, since the pandemic was particularly prevalent there at the time. iii. Further objectives after market approval e.g. targeted production capacity, cooperation with licensees and other partners (scale-up and scale-out) We aim at a targeted production capacity of 1.2 billion doses per year, starting from 2021. The manufacturing network will comprise Pfizer sites and partners in the US and EU, BioNTech sites and contractors in Germany/ the EU. Joint commercialization and distribution will use the infrastructure of BioNTech and its established partners, with the following allocation of regional distribution responsibilities (with BioNTech as marketing authorization holder): - BioNTech: Germany, Turkey - Fosun Health: China (incl. Hong Kong SAR, Macau SAR and Taiwan) - Pfizer: Rest of the world

3. State of the art; previous work a. Quality and development status of the preparatory work Please provide a brie summary o the state o the art including the rational or your vaccine development strategy and a comparative analysis to other approaches in the global COV D-19 vaccine pipeline (with a special ocus on speed e icacy sa ety production volume) hen make more detailed statements on the ollowing points – Global COVID-19 vaccine pipeline: There are currently no vaccines to prevent SARS-CoV-2 infection or the disease it causes, COVID-19 (Habibzadeh & Stoneman 2020). A large number of development programs are under way. A number of companies are pursuing mRNA-based vaccine programs. The other approaches can be grouped in the categories of viral vectors, DNA vaccines, protein-based and inactivated/ attenuated virus developments. While the WHO list >100 programs, only very few have made progress into clinical development (see chart below). Positioning of mRNA-based vaccine technologies within global COVID-19 pipeline: Novel mRNA technologies rely on using the cell’s own machinery to produce therapeutically relevant proteins - a highly versatile, multi-purpose approach. Whereas there is still no mRNA-based drug on the market, pharmaceutical development is advancing rapidly and on a global scale, driven by companies such as BioNTech, Moderna and CureVac. By encoding selected antigens from pathogens and thereby stimulating a natural immune response, the potential use of mRNA as a prophylactic vaccine has been shown in several clinical studies over the past decade. RNA-based vaccines are manufactured via a cell-free in vitro transcription process, which allows an easy and rapid production and the prospect of producing high numbers of vaccination doses within a shorter time period than achieved with traditional vaccine approaches. This capability is pivotal to enable the most effective response in outbreak scenarios. An LNP-formulated RNA based vaccine would provide one of the most flexible, scalable and fastest approaches to provide protection against the emerging viruses like SARS-CoV-2 (Rauch et al. 2018; Sahin et al. 2014). Based on the large body of peer-reviewed publications discussing the clinical and non-clinical data for 6

mRNA vaccines in development, and our own extensive knowledge and experience in this area, we have identified four key benefits of mRNA vaccines over other approaches: - Speed: A key advantage of mRNA vaccine platforms lies within the timely and effective response to emerging threats, since the manufacturing process differs only slightly between molecules (mainly within nucleotide sequences) and manufacturing processes and facilities can rapidly be adapted to produce GMP quality material for novel antigens. - Safety: RNA occurs naturally in the body, is metabolized and eliminated by the body’s natural mechanisms, does not integrate into the genome, is transiently expressed, and therefore is considered safe. Unlike live attenuated vaccines, RNA vaccines do not carry the risks associated with infection and may be given to people who cannot be administered live virus (eg, pregnant women and immunocompromised persons). - Efficacy: Vaccination with RNA in general generates robust immune responses as RNA not only delivers the vaccine antigen, but also has intrinsic adjuvanticity. mRNA-based vaccines have shown the potential to elicit strong neutralizing antibody responses and significant T cell responses, with a TH1 phenotype - a combination of immune characteristics thought to potentially maximize the probability of protection and minimize potential for disease enhancement. - Production Volume: As described above, the production of RNA requires only a single development and manufacturing platform, irrespective of the encoded pathogen antigens. Thus, RNA has the potential of rapid, cost-efficient, high-volume manufacturing and flexible stockpiling (long term storage of low-volume libraries of frozen plasmid and unformulated RNA, which can be rapidly formulated and distributed). Comparative Analysis of BNT162 vaccines: The BioNTech-Pfizer COVID-19 vaccine development program (see graph below) is based on BioNTech’s proprietary mRNA-based technology platforms and extensive knowledge in vaccine development for infectious diseases, which has been gained through several development programs over the last decade. Our approach is distinguishable from other COVID-19 mRNA approaches by the following main elements: - Diversified development approach: At BioNTech, there are three different RNA platforms under development, namely non-modified uridine containing mRNA (uRNA), nucleoside- modified mRNA (modRNA), and self-amplifying mRNA (saRNA). Each of these platforms displays unique features, and the probability of success for the COVID-19 program is highly increased by assessing 4-5 vaccine candidates in phase 1/2 trials (BNT162 vaccines), which are based on a selection from all three technology platforms and encoding for several 7

variants of the presumed protective antigen (full-length spike protein, mutated version, and receptor-binding domain). - Acceleration of development timeline: In order to prevent further COVID-19 infections as soon as possible, a rapid clinical development strategy has been our primary objective. We have initiated clinical trials in Germany and the US in parallel, accelerating timelines for subject recruitment and increasing potential for global approval; based on the assumption that the PEI is the most experienced regulatory agency with RNA vaccines and the FDA the most widely accepted approval mechanism in other countries. Additionally, we are designing our clinical trial protocols to aim at early accelerated / conditional approval (US & EU), and started extensive and continuous up-scaling activities as early as Feb 2020 (in parallel to preclinical development). Whereas we are convinced that the various companies active in developing an mRNA-based COVID-19 vaccine will complement each other in terms of global vaccine supply, this early commitment to a full-on COVID-19 program has enabled us to gain the following head start and competitive advantage: - Speed: BioNTech was the first European company with mRNA technology to enter clinical development (start of phase 1/2 trial in Germany as early as April 2020, shortly after Moderna in the US). The first data from our phase 1/2 trial program was reported in June and further data will be reported in July. We expect to enter phase 2b/3 development with our chosen candidate and dosing regimen in late July. BioNTech was thus one of the fastest companies overall to react to the crisis (initiation of pre-clinical work in Feb 2020) and the BioNTech-Pfizer vaccine program is now one of the most advanced COVID-19 programs world-wide (see chart above). - Safety: All three RNA platforms have been tested in more than a dozen non-clinical GLP safety studies and, for uRNA and modRNA, there is pre-existing clinical safety data. The RNA is delivered by protein-free lipid nanoparticles (LNPs), a potent and generally well- tolerated RNA delivery system. More than 40 subjects have been dosed in the ongoing phase 1/2 COVID-19 vaccine trials and no serious adverse events (SAEs) have been reported / no participants withdrew due to adverse events (AEs). Local reactions and systemic events were dose-dependent, generally mild to moderate, and transient. No serious adverse events were reported. (Published at https://www.medrxiv.org/content/10.1101/2020.06.30.20142570v1.full.pdf) - Clinical efficacy: The core innovation of BioNTech is based on in vivo delivery of a pharmacologically optimized, antigen-encoding RNA to induce robust neutralizing antibodies and a concomitant T cell response to achieve protective immunization with minimal vaccine doses (Vogel et al. 2018; Moyo et al. 2019; Pardi et al. 2017). We believe that we have strong preliminary data for our BNT162 vaccines in primates, have just released initial data from our US trial, and will be reporting additional phase 1/2 immunogenicity data at the end of June/July. Preliminary data showed that all subjects who received 10 µg or 30 µg of BNT162b1 had, at day 28, significantly elevated RBD-binding IgG antibodies as well as SARS-CoV-2 neutralizing antibodies with geometric mean titers mean titers (GMT) at or above the levels observed in convalescent sera. (Published at https://www.medrxiv.org/content/10.1101/2020.06.30.20142570v1.full.pdf) - Production Volume: BioNTech has profound expertise in production-process development for various RNA chemistries and formulations. 8

i. Clinical trial Please provide a detailed description o the current development stage Please provide in ormation on the choice o vaccine technology potency test and validation potential as well as relevant preclinical data (immunogenicity toxicology and sa ety in animal models) and estimated dose range or clinical use Please attach the relevant documents rom the consultations with the responsible regulatory authority (scienti ic advice) via the attachment unction he same applies i the approval or a phase or / clinical trial has already been granted Current development stage: As an integrated part of this project and in collaboration with Pfizer, we are conducting one of the most advanced SARS-CoV-2 clinical trial programs in the world, with two clinical phase 1/2 trials ongoing in Germany and in the US, testing four different mRNA vaccine candidates in humans in parallel, and allowing an informed selection of the most promising candidate for late stage clinical development. Phase 2/3 efficacy trials are expected to start in late July with up to 30,000 individuals and we anticipate that we will have first results by September. In parallel, BioNTech and Pfizer are actively scaling up manufacturing capacity and distribution infrastructure and we expect to begin to supply vaccine doses globally from October onwards (emergency and pandemic supply). Choice of vaccine technology: BioNTech has used three different RNA platforms for the development of BNT162 vaccine candidates: RNA which contains the standard nucleoside uridine (uRNA), nucleosidemodified RNA (modRNA), in which uridine is replaced by the nucleoside pseudo- uridine; and self-amplifying RNA (saRNA), which also contains uridine nucleosides (see figure below). Overview of the three RNA platforms The RNA vacc ne mo ecu es are capped, conta n open read ng frames (ORFs,) f anked by the untrans ated reg ons (UTR), and have a po yA-ta at the 3 end. The ORF of the uRNA and modRNA vectors encode the vacc ne ant gen. The saRNA has two ORFs. The f rst ORF encodes an a phav rus-der ved RNA-dependent RNA 9

po ymerase (rep case), wh ch upon trans at on med ates se f-amp f cat on of the RNA. The second ORF encodes the vacc ne ant gen. The utility of each of these RNA platforms for the development of infectious disease vaccines is supported by various non-clinical studies that demonstrated the efficient induction of potent neutralizing antibody and T-cell responses against a variety of viral pathogens including influenza, Ebola, human immunodeficiency virus (HIV), and Zika virus (Vogel et al. 2018; Moyo et al. 2018; Pardi et al. 2017). These three RNA platforms display various features, as follows: uRNA is associated with high intrinsic adjuvanticity, modRNA with blunted innate immune sensor activating capacity and thus augmented antigen expression, and saRNA with the potential to reach higher amounts of protein per injected RNA template, thereby enhancing immunogenicity and potentially decreasing the dose needed for efficient protection. The structural elements of the vector backbones of BNT162 vaccine candidates are optimized for prolonged and strong translation of the antigen-encoding RNA component. The potency of BNT162 vaccine candidates is further optimized by encapsulation of the RNA component into LNPs, which protect the RNA from degradation by RNAses and enable transfection of host cells after IM delivery. Due to RNA’s inherent adjuvant activity mediated by binding to innate immune sensors such as toll like receptors, RNA-LNP vaccines induce a robust neutralizing antibody response and a concomitant T-cell response resulting in protective immunization with minimal vaccine doses. As target antigen, the coronavirus spike protein and its receptor-binding domain (RBD) were chosen. Coronaviruses like SARS-CoV-2 are a (+)ssRNA enveloped virus family that encode for a total of four structural proteins. Within these four structural proteins, the spike glycoprotein (S protein) is the key target for vaccine development. Similar to the influenza virushemagglutinin (HA), the S protein is responsible for receptor-recognition, attachment to the cell, viral envelope fusion with a host cell membrane, and genomic release driven by the S protein conformation change, leading to the fusion of viral and hosts cell membranes. The S protein is cleaved by host proteases into the S1 and S2 subunits. While S2, with its transmembrane domain, is responsible for membrane fusion, the S1 domain with its C- terminal receptor-binding domain (RBD) recognizes the host receptor and binds to the target host cell. SARS-CoV and SARS-CoV-2 have similar structural properties and bind to the same host cell receptor, angiotensin converting enzyme 2 (ACE-2) (Zhou et al. 2020). The S protein is not only pivotal for host cell recognition and entry, but also for the induction of virus neutralizing antibodies by the host immune system (Zakhartchouk et al. 2007; Yong et al. 2019). Some monoclonal antibodies against the S protein, particularly those directed against the RBD, neutralize SARS-CoV and Middle East respiratory syndrome (MERS-)-CoV infection in vitro and in vivo (Hulswit et al. 2016). Targeting the S protein, as well as its S1 cleavage fragment or the RBD alone, with vaccines is sufficient to induce neutralizing immune responses (Al-Amri et al. 2017). Dose range for clinical use: 10










