2.Bescheid.Anlage11_VB_IDT
Dieses Dokument ist Teil der Anfrage „Vereinbarungen mit den Firmen BioNTech SE, CureVac AG und IDT Biologika GmbH in Bezug auf Impfstoffe gegen SARS-CoV-2“
'DZIF ÖIDT Project Description for preclinical, clinical and CMC development of the live recombinant COVID-19 vector vaccine MVA-SARS-2-S Deutsches Zentrum für Luft- und Raumfahrt e. V. -Projektträger — Gesundheitsforschung — Heinrich-Konen-Straße 1 53227 Bonn Applicant: IDT Biologika GmbH Am Pharmapark 06861 Dessau-Rosslau Germany Consortium Members: DZIF (German Center for Infection Research), Inhoffenstraße 7, D- 38124 Braunschweig, Institute for Infectious Diseases and Zoonosis, Ludwig-Maximilians-University München, Veterinärstr. 13, D-80539 München; Institute for Virology, Philipps-University Marburg, Hans-Meerwein-Str 2, D-35043 Marburg Zentrum für Innere Medizin, |. Medizinische Klinik und Poliklinik (Gastroenterologie mit Sektionen Infektiologie und Tropenmedizin), Universitätsklinikum Hamburg- Eppendorf, Martinistrasse 52, D-20246 Hamburg. Abteilung für Infektions- und Tropenmedizin (Tropeninstitut), Klinikum der Ludwig- Maximilians-Universität (LMU), Leopoldstrasse 5, D-80802 München Medizinische Klinik, Innere Medizin VII, Institut für Tropenmedizin, Reisemedizin, Humanparasitologie, Kompetenzzentrum Tropenmedizin Baden-Württemberg, Wilhelmstraße 27, D-72074 Tübingen. Date 10.July 2020 IDT / Scientific Contact IDT Managing Director

Sonderprogramm zur Beschleunigung von Forschung und Entwicklung dringend benötigter Impfstoffe gegen SARS-CoV-2 CONFIDENTIAL IDT INFORMATION Table of content 12 ı TMUOÄUEREN er ee nen ee date Fass nn eg 3 a. Brief description ofthe applying company IDT Biologika GmbH «aan 3 Br. EEE ETTRRT nei eig Dem na ETTE 4 2 BIER VES anna EEE Hgg age finale SEE 5 a. Scientific and/or technical working objectives ofthe projact...uucanacaccancanannannnnnnnnnnnnnn 5 i. Objectives of clinical development, including objectives for adjustments/ expansions of manufacturing, processing and filling capacities within the clinical trial process .......uuccucacancncnnnnnnnnnnnnn nn 5 ii. Objectives regarding collaboration with development and production partners during clinical trials ...... 5 ii. Further objectives after market approval e.g. targeted production capacity, cooperation with licensees and other partners (scale-up and scale-out) ........uuusnaenennennenenennnnnnnnnnnnnnannnnnnnnnnnnnnnn 5 3 State of the art; previous work .....uuessessusensnnnunnnnnunnennnnnnnnnnnnnnennnnnnannnnnnnnnnnannnnnnnnnnnnnnnnnnnn nn 6 a Quality and development status of the preparatory work..unanauaseanuacannanunnunnanunnnnnnntnnnnnnn nn 6 | CHRIE IA en nreaigssssts: tape ee ae ee N RE Ten eete 9 ü Vacslfe HFOHUCHON Gun ren ee an een en re 16 ii. Declaration on intellectual property rights b Previous work of the applicant..........uaesece.. 4. Detailed description of the work plan MIESIORE'SCHSAUI Eine eanschriesne a eERRE e Plans to accelerate the development/expansion of clinical trial and production capacities .................... 28 i. Acceleration of development.........ueeneeeneesesensnenennmnennennnansennnannnnnnnnennnannnnnnnnnnnnnnn nn 28 ii. Expansion of clinical trial capacities........uunanasasnnsnennennnnnnnnnnnnnnnnnnnnnnnnne 28 Ni, Extpansion ofproduetän Bapaditiäs.. unseren aan 29 c Risk assessment, mitigation and avoidance......uunaaasnesnenanannnnnnnnnnnnnnnnnunnnnnnnnnn nn 34 5. Exploitation and Dissemination Plan......uuecaasanasnannanensenennennennennnnnnnannnnnnnnnnnnnnnnnnnnnnnnn nn 38 ab, BIIEFOVERMEW.: u ER RAN eipesgee innen For A ET ee 38 b. Aspects of economic, scientific and technical exploitation prior to marketing authorisation ..................... 38 c. Aspects of exploitation after marketing authorisation 6. Division of labour/cooperation with third parties.................. 7. _ Financing ofthe project, cost estimates..........unnaensnnnnnnnnnenennennnnnnnnnnnnnnnnnnnnnnannnnnnnnnnnnnnnnnnennnnennn Annex I - MVA-SARS-2-S Vaccine Development Project Gantt Chart Annex II - Synopsis for Clinical Investigations ...........uuunasaeanneannneneneenmnennnnnnnnnnnnnnnannnnnnnnnnnnnnnnnnnan I1.1. Synopsis of MVA-SARS-2-S Phase la clinical trial................000000000..nnn. II.2. Synopsis of CERMEL MVA-SARS-2-S Phase I clinical trial... 11.3. Synopsis of VGCARE MVA-SARS-2-S Phase I clinical trial.................0u0..nn 11.4. Synopsis of MVA-SARS-2-S Phase Il clinical trial.........uaaanannannnnnnnnnnnnnnnnnnnnn 54 11.5. Synopsis of MVA-SARS-2-S Phase Ilb clinical trial............uucaanananannnnnnnnnnn 57 11.6. Synopsis of MVA-SARS-2-S Phase Ill clinical trial........unaccaannnnnnnnnn 60

Sonderprogramm zur Beschleunigung von Forschung und Entwicklung dringend benötigter Impfstoffe gegen SARS-CoV-2 CONFIDENTIAL IDT INFORMATION 1. Introduction a. Brief description of the applying company IDT Biologika GmbH IDT Biologika GmbH is a European and US Food and Drug Administration (FDA) registered pharmaceutical manufacturer (No: FEI3005001757). IDT manufacturing operations are governed under United States, German and European Union applicable laws and current Good Manufacturing Practice. IDT holds a Manufacturing License and GMP Certificate issued by Landesverwaltungsamt (provincial administrative office) Saxony-Anhalt, Germany. IDT has vaccine development and manufacturing sites in Germany and in the US. IDT has almost 100 years history in development and manufacturing of biological and pharmaceutical products for veterinary and human use. IDT received market authorization for a range of innovative veterinary vaccines atthe EMA and supported customer and partner development programs and successful applications for IMPD’s, IND’s and Market Authorization for innovative human vaccines at the EMA and FDA. Since 1997 IDT provided more than 40 innovative recombinant vaccine candidates for Phase 1,2 and 3 clinical studies worldwide. IDT has more than 30 own approved by European and other Agencies veterinary vaccines on the market. The veterinary business was sold in 2019 to CEVA. Since that point of time IDT has increased the strategic focus on development and manufacturing of biological immunological products for human use. Currently, a range of approved pharmaceutical products and 3 commercial vaccines are manufactured at IDT. At this stage IDT develops in collaboration with DZIF institutions, Erasmus Medical Centre the clinical partner CR2O the MERS Coronavirus vaccine MVA-MERS-S. That project is funded by CEPI. Development of the COVID 19 vaccine candidate will be based on the technology platform developed for the MERS Coronavirus vaccine MVA-MERS-S (CEPI Funding). The development Consortium including following institutions: e IDT Biologika GmbH, Am Pharmapark, 06862 Dessau- Roßlau, Germany; e DZIF (German Center for Infection Research), Inhoffenstraße 7, 38124 Braunschweig, Germany; « Institute for Infectious Diseases and Zoonosis, Ludwig-Maximilians-University München, Veterinärstr. 13, D-80539 München; ° Institute for Virology, Philipps-University Marburg, Hans-Meerwein-Str 2, 35043 Marburg e Zentrum für Innere Medizin, I. Medizinische Klinik und Poliklinik (Gastroenterologie mit Sektionen Infektiologie und Tropenmedizin), Universitätsklinikum Hamburg- Eppendorf, Martinistrasse 52, 20246 Hamburg. e Abteilung für Infektions- und Tropenmedizin (Tropeninstitut), Klinikum der Ludwig- Maximilians-Universität (LMU), Leopoldstrasse 5, 80802 München e Medizinische Klinik, Innere Medizin VII, Institut für Tropenmedizin, Reisemedizin, Humanparasitologie, Kompetenzzentrum Tropenmedizin Baden-Württemberg, Wilhelmstraße 27, 72074 Tübingen.

Sonderprogramm zur Beschleunigung von Forschung und Entwicklung dringend benötigter Impfstoffe gegen SARS-CoV-2
CONFIDENTIAL IDT INFORMATION
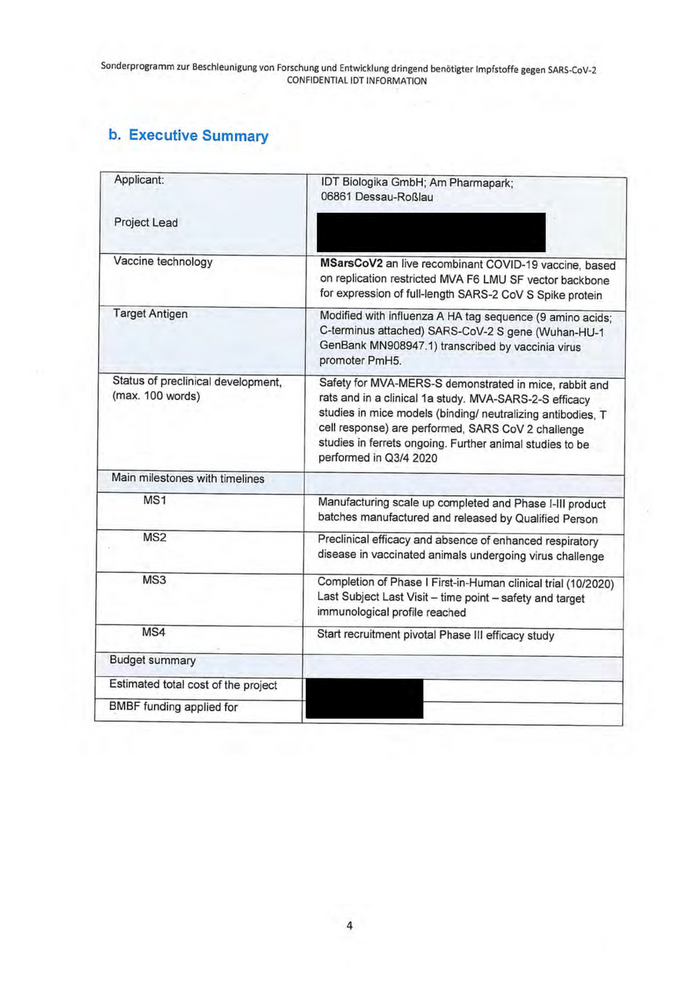
b. Executive Summary
Applicant: IDT Biologika GmbH; Am Pharmapark;
06861 Dessau-Roßlau
Vaccine technology MSarsCoV2 an live recombinant COVID-19 vaccine, based
on replication restricted MVA F&6 LMU SF vector backbone
for expression of full-length SARS-2 CoV S Spike protein
Target Antigen Modified with influenza A HA tag sequence (9 amino acids;
C-terminus attached) SARS-CoV-2 S gene (Wuhan-HU-1
GenBank MN908947.1) transcribed by vaccinia virus
promoter PmH35.
Safety for MVA-MERS-S demonstrated in mice, rabbit and
rats and in a clinical 1a study. MVA-SARS-2-S efficacy
studies in mice models (binding/ neutralizing antibodies, T
cell response) are performed, SARS CoV 2 challenge
studies in ferrets ongoing. Further animal studies to be
performed in Q3/4 2020
MS1 Manufacturing scale up completed and Phase |-IIl product
batches manufactured and released by Qualified Person
MS2 Preclinical efficacy and absence of enhanced respiratory
disease in vaccinated animals undergoing virus challenge
Completion of Phase | First-in-Human clinical trial (10/2020)
Last Subject Last Visit — time point — safety and target
immunological profile reached
Start recruitment pivotal Phase Ill efficacy study
Estimated total cost of the project | = = 5
Status of preclinical development,
(max. 100 words)
Sonderprogramm zur Beschleunigung von Forschung und Entwicklung dringend benötigter Impfstoffe gegen SARS-CoV-2 CONFIDENTIAL IDT INFORMATION 2. Objectives a. Scientific and/or technical working objectives of the project The objective of this application is development and conditional Market Approval Authorization for an innovative live recombinant viral vaccine for active immunization of adults and elderly considered at-risk for protection against clinical SARS-2-CoV infections. i. Objectives of clinical development, including objectives for adjustments/ expansions of manufacturing, processing and filling capacities within the clinical trial process The objective for clinical development is creation of a broad basis for multi centric clinical testing and immunological monitoring of subjects immunized with the innovative MVA-SARS- 2-S vaccine in Germany, at one site in Africa and one site in Asia. In parallel the manufacturing technology should be up-scaled to commercial scale for supply of such vaccine batches for Phase Il and Ill clinical trials. For manufacturing and quality control testing fast track technologies should be used to facilitate shortest possible manufacturing cycles and assure comparability of clinical trial vaccine batches to commercial batches, IDT Biologika GmbH will increase in parallel manufacturing capacities for vaccine Drug Substance and filling capacities for Drug Product production at least nV .- SARS-2-S vaccine per year. ii. Objectives regarding collaboration with development and production partners during clinical trials The urgent need for a vaccine and scientific understanding of COVID-19 requires a coordinated collaboration between virological, preclinical, immunological and clinical teams and vaccine manufacturing and characterization. Our Consortium aims at an efficient real time information policy and regular scientific and management review consultations for fast track development and decisions. It is of utmost importance to include any knew knowledge and information into the existing data package for filing the Market Approval Authorization and possible required adjustments in parallel to the planned investigations. Filing of the MAA should be performed in close collaboration and in parallel to IMPD applications, preclinical and clinical progress. Regular scientific meeting with regulatory agencies (PEI; EMA) are substantial part of our development program and support the fast track development approach, iii. Further objectives after market approval e.g. targeted production capacity, cooperation with licensees and other partners (scale-up and scale-out) We understand the risks for development of new manufacturing capacities for an unknown new product. Since IDT is well accepted in contract development and manufacturing area that also requires intensive collaboration with partners, we are open for transfer of our technology to other companies for further increase of manufacturing capacities and scale. In case of non-satisfactory results for our vaccine candidate we provide our capacities and knowledge for manufacturing of any other promising COVID-19 vaccines. 5

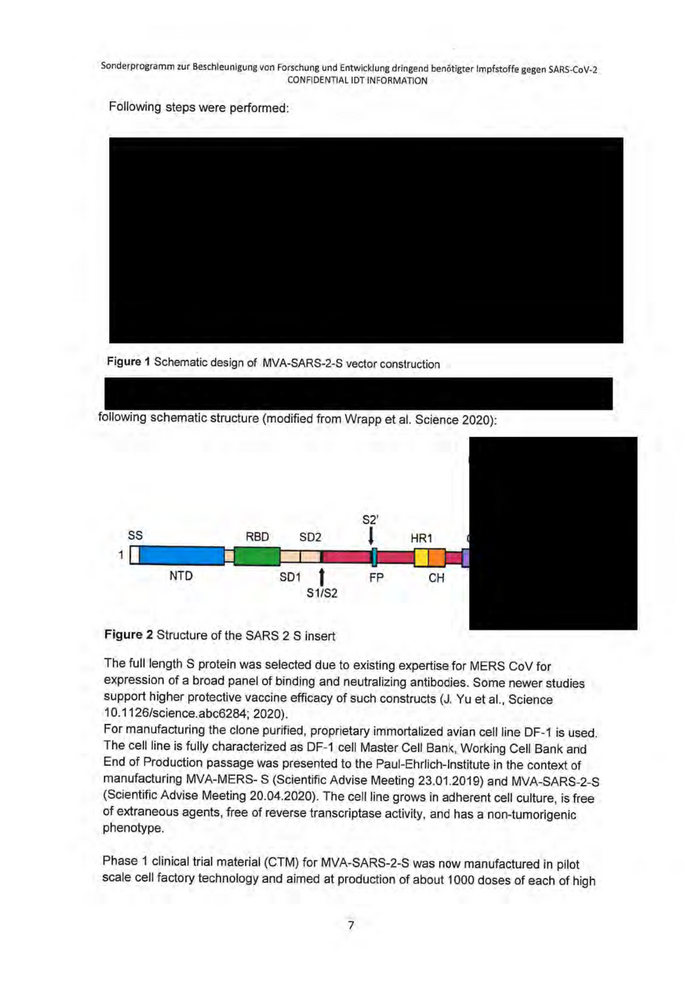
Sonderprogramm zur Beschleunigung von Forschung und Entwicklung dringend benötigter Impfstoffe gegen SARS-CoV-2 CONFIDENTIAL IDT INFORMATION 3. State of the art; previous work a. Quality and development status of the preparatory work General information MVA is a vaccine virus and non-replicating vector capable of eliciting an immune response to relevant transgene products upon expression in human target tissues. It is a particularly safe vaccine platform that has never been associated with severe adverse events (SAEs) in humans, including immune compromised individuals. DZIF and IDT developed and manufactured aMERS CoV vaccine that was intensively tested in preclinical studies and in a phase 1 a clinical trial (Eudra CT number: 2014- 003195-23). That vaccine candidate was manufactured in primary chicken embryo fibroblasts (CEF). The peer reviewed published phase la clinical trial data for MVA-MERS-S (CEF) (Koch T et al., 2020) highlighted following characteristics: * Homologous prime-and-boost immunizations with MVA-MERS-S revealed a benign safety profile with only transient mild-to-moderate reactogenicity ° Participants experienced no severe or serious adverse events (AE) ° Local reactions (e.g. swelling, erythema, pain), headache and fatigue were the most common AE and seen in 69% (18/26), 62% (16/26) and 65% (17/26), respectively « AIIAE resolved swiftly (median within one day) and without sequelae ° Following booster immunization, 87% (20/23) of all vaccinees showed seroconversion using an MERS-CoV-S1-ELISA « Antibody titers correlated with MERS-CoV-specific neutralizing antibodies ° MERS-CoV-S-specific T-cell responses were detected in 87% cases (20/23) ° MVA-MERS-S applied in low dose (1x107 pfu/0,5ml) and high dose (1x10° pfu/0,5ml) prime boost regimen elicited comparable immune responses Manufacturing technologies in CEF are not sufficientiy scalable and highly variable. The project was further developed within a CEPI Grant for implementation of a cell line based scalable manufacturing technology and continuation of clinical studies in Phase 1b and phase 2. The result of that development, an innovative and scalable vaccine manufacturing technology was now implemented at IDT and provides the basis for large-scale manufacturing of significant higher numbers of vaccine doses with significant improved impurity profile in terms of host cell residuals. The MVA- SARS- 2- S vaccine candidate was developed by Prof. Gerd Sutter at LMU Munich. The recombinant vector was generated in GMP qualified DF-1 cells and media provided by IDT. The vaccine candidate was in a preliminary test investigated for genetic stability. Further studies including 5 consecutive passages in DF-1 cells and full genome sequencing are ongoing. A modified synthetic S gene sequence from SARS-CoV-2 isolate Wuhan-HU-1 (GenBank MN908947.1) with a tag sequence encoding nine amino acids (YPYDVPDYA,; aa 98-106; Wilson IA et al. Cell 1984) from influenza A virus hemagglutinin (HAtag) attached at the C terminus was used and inserted into the genome of MVA F6 LMU SF under transcriptional control of vaccinia virus promoter PmH5.' ! The tag was needed during construction to detect expression as high affinity S antibodies were not yet available then. Our risk analysis identifies the tag as an innocuous sequence not associated with increased risk. 6

Sonderprogramm zur Beschleunigung von Forschung und Entwicklung dringend benötigter Impfstoffe gegen SARS-CoV-2 CONFIDENTIAL IDT INFORMATION Following steps were performed: Figure 1 Schematic design of MVA-SARS-2-S vector construction following schematic structure (modified from Wrapp et al. Science 2020): 52’ ss RBD sD2 | ı U 1 | NTD spot f FP 51852 Figure 2 Structure of the SARS 2 S insert The full length S protein was selected due to existing expertise for MERS CoV for expression of a broad panel of binding and neutralizing antibodies. Some newer studies support higher protective vaccine efficacy of such constructs (J. Yu etal., Science 10.1126/science.abc6284; 2020). For manufacturing the clone purified, proprietary immortalized avian cell line DF-1 is used. The cell line is fully characterized as DF-1 cell Master Cell Bank, Working Cell Bank and End of Production passage was presented to the Paul-Ehrlich-Institute in the context of manufacturing MVA-MERS- S (Scientific Advise Meeting 23.01.2019) and MVA-SARS-2-S (Scientific Advise Meeting 20.04.2020). The cell line grows in adherent cell culture, is free of extraneous agents, free of reverse transcriptase activity, and has a non-tumorigenic phenotype. Phase 1 clinical trial material (CTM) for MVA-SARS-2-S was now manufactured in pilot scale cell factory technology and aimed at production of about 1000 doses of each of high
Sonderprogramm zur Beschleunigung von Forschung und Entwicklung dringend benötigter Impfstoffe gegen SARS-CoV-2 CONFIDENTIAL IDT INFORMATION and low dose formulations. Such batches will be released for start of Ph1 clinical trials in Q3 2020. IDT initiated implementation of a large scale drug substance manufacturing line that should a titer 1x10”)to assure the required product quantities for Phase Il and Ill clinical trials. The line will be operational in September 2020. Formulation and filling capacities for the clinical development can be assured. Large-scale commercial manufacturing requires setup of additional capacities as described below. We envisage use of MVA-SARS-2-S as a homologous prime-and-boost vaccine candidate in risk groups including healthcare workers, elderly and comorbid patients. Compared to other vaccine projects we believe that the prime-boost application facilitates induction of more homogeneous immune responses and may also support the boost vaccination of previous single dose recombinant adenoviral COVID-19 vaceines. Investigation of such heterologous prime/boost is planned in the clinical development program. Toxicity studies During a Scientific Advice Meeting on 24.4.2020 Paul-Ehrlich-Institute waived the performance of repeat-dose-toxicity study with MVA-SARS-2-S based on the following grounds: First, MVA-MERS-S was tested extensively in GLP tox studies in both rat and rabbit. Dosing was at Full Human Dose and n+1 doses were administered in comparison to the planned clinical schedule. Neither of the two studies revealed product-related, dose- limiting toxicities. Second, the early immunogenicity testing of MVA-SARS-2-S in mice will involve toxicity endpoints. The rationale for the extensive toxicity testing of MVA-MERS-S was as follows. In the Wistar rat study, chicken embryonic fibroblast derived vaccine material (for phase la) was used. Since phase Ib clinical trial material of MVA-MERS-S was manufactured on DF-1 cells, it was planned to repeat the study for DF-1 derived virus material. We performed a GLP-compliant toxicology study in in the accepted New Zealand White rabbit toxicology model. Following contemporary vaccine development guidelines by WHO, EMA and FDA the MVA-MERS-S has been tested as a full human dose in a timely compressed n+1 vaccination schedule (a total of 4 doses) including a follow-up or recovery period. Dosed animals have been followed up into clinical observation, hematology, clinical biochemistry, urinalysis, blood coagulation and eventually histopathology. For this study an experimental vaccine batch with a relatively high level of impurities (host cell protein and DNA content) and a simulated high content of excipients was used (Tox batch). This approach covers any future process residual or impurity levels in order to obviate the need for repeat toxicology studies as we move along. This preclinical test strategy in conjunction with the concrete study design of the pivotal GLP toxicology study has been presented as a Scientific Advice to the Competent Authority of Germany, the Paul-Ehrlich- Institute in January 2019. The final results of the study are presented in the preclinical report (Study No. 604.362.5803): no mortality, no clinical observation and no adverse findings were made in any of the treatment groups in any animal and results were

Sonderprogramm zur Beschleunigung von Forschung und Entwicklung dringend benötigter Impfstoffe gegen SARS-CoV-2
CONFIDENTIAL IDT INFORMATION
comparable to the CEF-derived material tested in Wistar rats. The NOAEL? of the DF-1
derived material has been defined to be a repeated dose of 2.4x10° pfu study medication.
i. Clinical trial
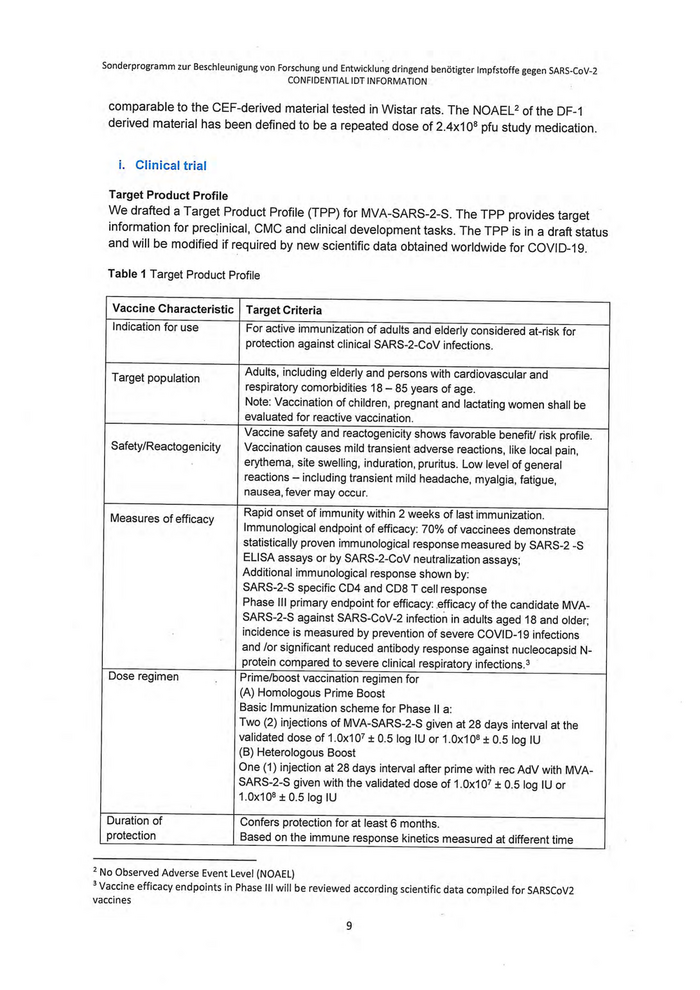
Target Product Profile
We drafted a Target Product Profile (TPP) for MVA-SARS-2-S. The TPP provides target
information for preclinical, CMC and clinical development tasks. The TPP is in a draft status
and will be modified if required by new scientific data obtained worldwide for COVID-19.
Table 1 Target Product Profile
Vaccine Characteristic | Target Criteria
For active immunization of adults and elderly considered at-risk for
protection against clinical SARS-2-CoV infections.
Indication for use
Adults, including elderly and persons with cardiovascular and
respiratory comorbidities 18 — 85 years of age.
Note: Vaccination of children, pregnant and lactating women shall be
evaluated for reactive vaccination.
Vaccine safety and reactogenicity shows favorable benefit/ risk profile.
Vaccination causes mild transient adverse reactions, like local pain,
erythema, site swelling, induration, pruritus. Low level of general
reactions — including transient mild headache, myalgia, fatigue,
nausea, fever may occur.
Target population
Safety/Reactogenicity
Rapid onset of immunity within 2 weeks of last immunization.
Immunological endpoint of efficacy: 70% of vaccinees demonstrate
statistically proven immunological response measured by SARS-2 -S
ELISA assays or by SARS-2-CoV neutralization assays;
Additional immunological response shown by;
SARS-2-S specific CD4 and CD8 T cell response
Phase Ill primary endpoint for efficacy: efficacy of the candidate MVA-
SARS-2-S against SARS-CoV-2 infection in adults aged 13 and older;
incidence is measured by prevention of severe COVID-19 infections
and /or significant reduced antibody response against nucleocapsid N-
protein compared to severe clinical respiratory infections.?
Prime/boost vaccination regimen for
(A) Homologous Prime Boost
Basic Immunization scheme for Phase Il a:
Two (2) injections of MVA-SARS-2-S given at 28 days interval at the
validated dose of 1.0x107 + 0.5 log IU or 1.0x10® + 0.5 log IU
(B) Heterologous Boost
One (1) injection at 28 days interval after prime with rec AdV with MVA-
SARS-2-S given with the validated dose of 1.0x107 + 0.5 log IU or
1.0x10® + 0.5 log IU
Measures of efficacy
Dose regimen
Confers protection for at least 6 months.
Based on the immune response kinetics measured at different time
Duration of
protection
? No Observed Adverse Event Level (NOAEL)
® Vaccine efficacy endpoints in Phase Ill will be reviewed according scientific data compiled for SARSCoV2
vaccines
Sonderprogramm zur Beschleunigung von Forschung und Entwicklung dringend benötigter Impfstoffe gegen SARS-CoV-2
CONFIDENTIAL IDT INFORMATION
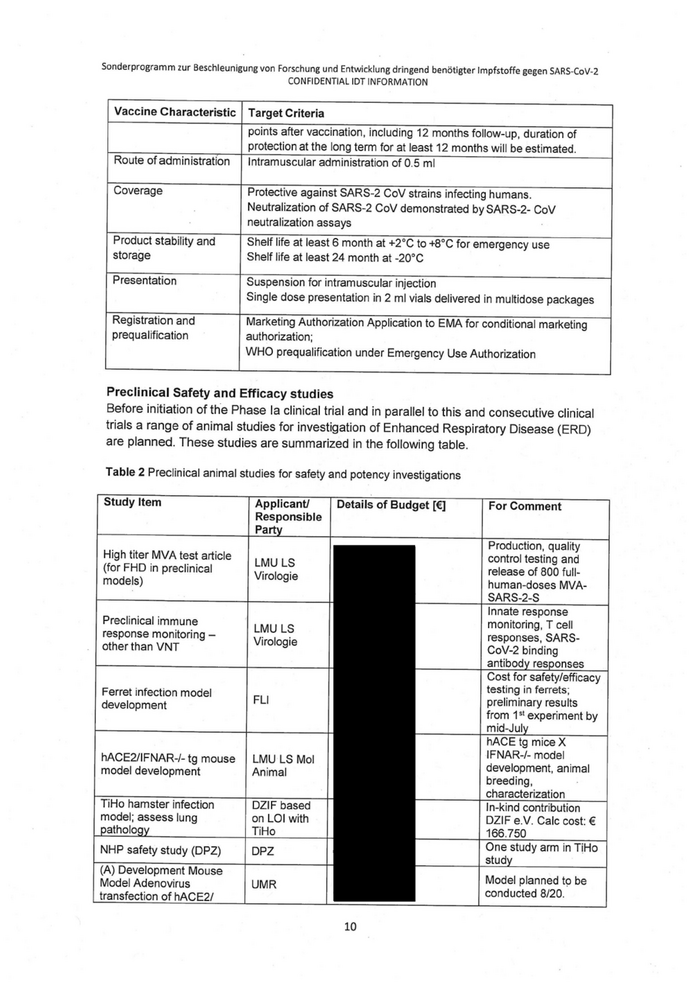
Vaccine Characteristic | Target Criteria
points after vaccination, including 12 months follow-up, duration of
Route ofadministration
protection at the long term for at least 12 months will be estimated.
Intramuscular administration of 0.5 ml
Product stability and
storage
Registration and
prequalification
Protective against SARS-2 CoV strains infecting humans.
Neutralization of SARS-2 CoV demonstrated by SARS-2- CoV
neutralization assays
Shelf life at least 6 month at +2°C to +8°C for emergency use
Shelf life at least 24 month at -20°C
Suspension for intramuscular injection
Single dose presentation in 2 ml vials delivered in multidose packages
Marketing Authorization Application to EMA for conditional marketing
authorization;
WHO prequalification under Emergency Use Authorization
Preclinical Safety and Efficacy studies
Before initiation of the Phase la clinical trial and in parallel to this and consecutive clinical
trials a range of animal studies for investigation of Enhanced Respiratory Disease (ERD)
are planned. These studies are summarized in the following table.
Table 2 Preclinical animal studies for safety and potency investigations
Applicant/
Responsible
Part
Production, quality
control testing and
release of 800 full-
human-doses MVA-
High titer MVA test article
(for FHD in preclinical
models)
LMU LS
Virologie
Innate response
monitoring, T cell
responses, SARS-
CoV-2 binding
antibody responses
Cost for safety/efficacy
testing in ferrets;
preliminary results
from 1°! experiment by
mid-Jul
hACE tg mice X
Preclinical immune
response monitoring —
other than VNT
Virologie
Ferret infection model
development
IFNAR-/- model
em | Eat Geveoamen anna
breeding,
characterization
In-kind contribution
DZIF e.V. Calc cost: €
166.750
stud
Model planned to be
conducted 8/20.
TiHo hamster infection DZIF based
model; assess lung on LOI with
patholog TiHo
NHP safety study (DPZ)
(A) Development Mouse
Model Adenovirus UMR
transfection of HMACE2/