2.Bescheid.Anlage14_VB_CureVac
Dieses Dokument ist Teil der Anfrage „Vereinbarungen mit den Firmen BioNTech SE, CureVac AG und IDT Biologika GmbH in Bezug auf Impfstoffe gegen SARS-CoV-2“
Application for Funding by German Federal Ministry of Education and Research ACcelerated and Expedited R&D of mRNA vaccine against SARS-CoV-2: ACE-mR-CoV Submitted by CureVac AG Friedrich-Miescher-Strasse 15 72076 Tübingen, Germany Project Lead: Submitted on July 14, 2020 - confidential – I

Project description: Table of content List of Abbreviations ............................................................................................................. IV List of Tables ....................................................................................................................... VII List of Figures ...................................................................................................................... VII 1. Introduction .................................................................................................................... 1 a. Brief description of the applying company/institution ................................................... 1 b. Executive Summary in tabular form ............................................................................ 1 2. Objectives ...................................................................................................................... 2 a. Scientific and/or technical working objectives of the project ........................................ 2 i. Objectives of clinical development, including objectives for adjustments/ expansions of manufacturing, processing and filling capacities within the clinical trial process.......... 5 ii. Objectives regarding collaboration with development and production partners during clinical trials .................................................................................................................... 6 iii. Further objectives after market approval e.g. targeted production capacity, cooperation with licensees and other partners (scale-up and scale-out) ......................... 7 3. State of the art; previous work ........................................................................................ 7 a. Quality and development status of the preparatory work ............................................. 7 i. Clinical trial .............................................................................................................. 8 ii. Vaccine production .................................................................................................12 iii. Declaration on intellectual property rights ...............................................................16 b. Previous work of the applicant ...................................................................................19 4. Detailed description of the work plan .............................................................................22 a. Milestone Schedule ...................................................................................................22 b. Plans to accelerate the development/expansion of clinical trial and production capacities..........................................................................................................................32 i. Acceleration of development ..................................................................................32 ii. Expansion of clinical trial capacities .......................................................................32 iii. Expansion of production capacities ........................................................................32 c. Risk assessment, mitigation and avoidance ...............................................................37 5. Exploitation and Dissemination Plan..............................................................................43 a. Brief overview ............................................................................................................43 b. Aspects of economic, scientific and technical exploitation prior to marketing authorisation .....................................................................................................................44 c. Aspects of exploitation after marketing authorisation .................................................46 6. Division of labour/cooperation with third parties .............................................................46 7. Financing of the project, cost estimates .........................................................................49 Own contribution, BMBF contribution, third-party financing ...............................................49 References: ..........................................................................................................................63 Annex 1 - Milestone Planning ...............................................................................................64 Annex 2 – Scientific Advises from Authorities .......................................................................64 Annex 3 – CureVac AG Risk Assessment Policy, Version 0.2 ..............................................64 II

Annex 4 - Investigator’s Brochure “mRNA based SARS-CoV-2 Vaccine IB", Version 2.0 .....64 Annex 5 – Clinical Study Protocol, Study CVnCoV-001 ........................................................64 III

List of Abbreviations AE Adverse Event AESI Adverse Event of Special Interest AP “Arbeitspaket” (Working Package) BMGF Bill & Melinda Gates Foundation CEPI Coalition for Epidemic Preparedness Innovations CI Confidence Interval CTA Clinical Trial Approval CMC Chemistry Manufacturing and Controls CMO Contract Manufacturing Organisation CVnCoV CureVac novel Coronavirus Vaccine CPE Cytopathic Effect CRO Contract Research Organisation DART Developmental And Reproductive Toxicology DNA Deoxyribonucleic Acid DP Drug Product DSMB Data Safety Monitoring Board EC Ethics Committee EEA European Economic Area EFD Embryo-Fetal Development EMA European Medicines Agency EP European EUL Emergency Use Listing FACS Fluorescence-Activated Cell Sorting FAIR Principles Findable, Accessible, Interoperable and Reusable Principles FAMHP Federal Agency for Medicines and Health Products FDA Food and Drug Administration FEED Fertility and Early Embryo Development FIH First In Human FLI Friedrich-Loeffler-Institut GAVI Global Alliance for Vaccines and Immunisation IV
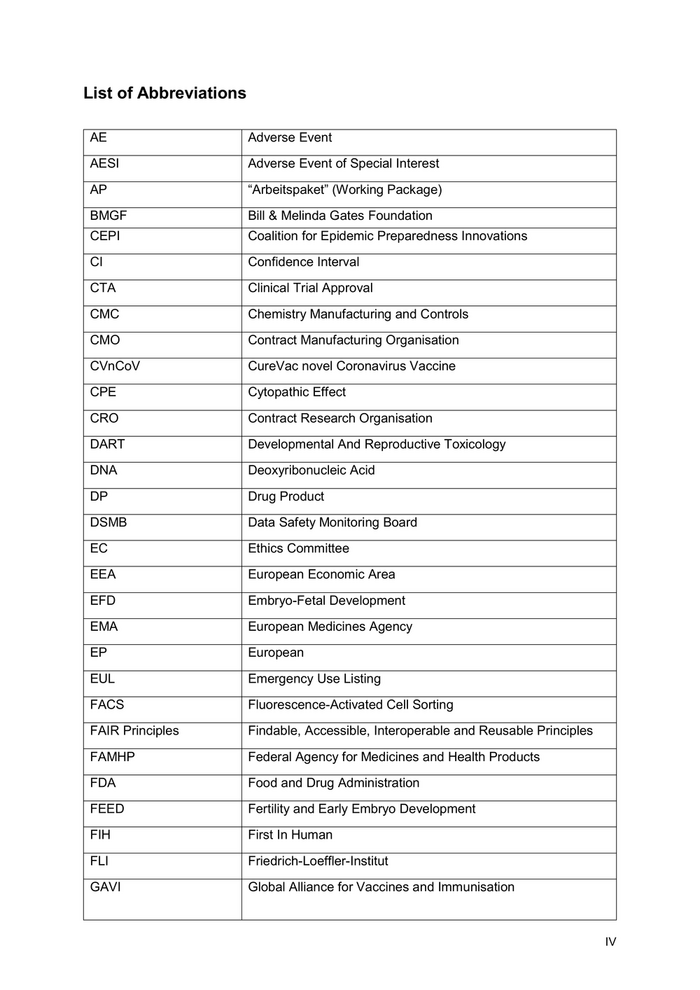
GLP Good Laboratory Practice GMP Good Manufacturing Practice H0 Hypotheses 0 HA Health Authorities IB Investigator Brochure lgG Immunoglobulin G IM intramuscular IND Investigational New Drug iSRC internal Safety Review Committee IVT In Vitro Translation KfW Kreditanstalt für Wiederaufbau LNP Lipid Nanoparticle MAA Marketing Authorization Approval MERS Middle East Respiratory Syndrome mRNA messenger Ribonucleic Acid MS Milestones n Number (as in Sample Size) N protein Nucleocapsid protein NHP Non-Human Primates ORF Open Reading Frame PBMC Peripheral Blood Mononuclear Cell pDNA plasmid DNA PCT Patent Cooperation Treaty PEI Paul-Ehrlich-Institute PPND Pre- and Postnatal Development PRA Pharmaceutical Research Associates R&D Research & Development RBD Receptor Binding Domain RNA Ribonucleic Acid ROW Rest of the World RSV Respiratory Syncytial Virus SAE Serious Adverse Event V
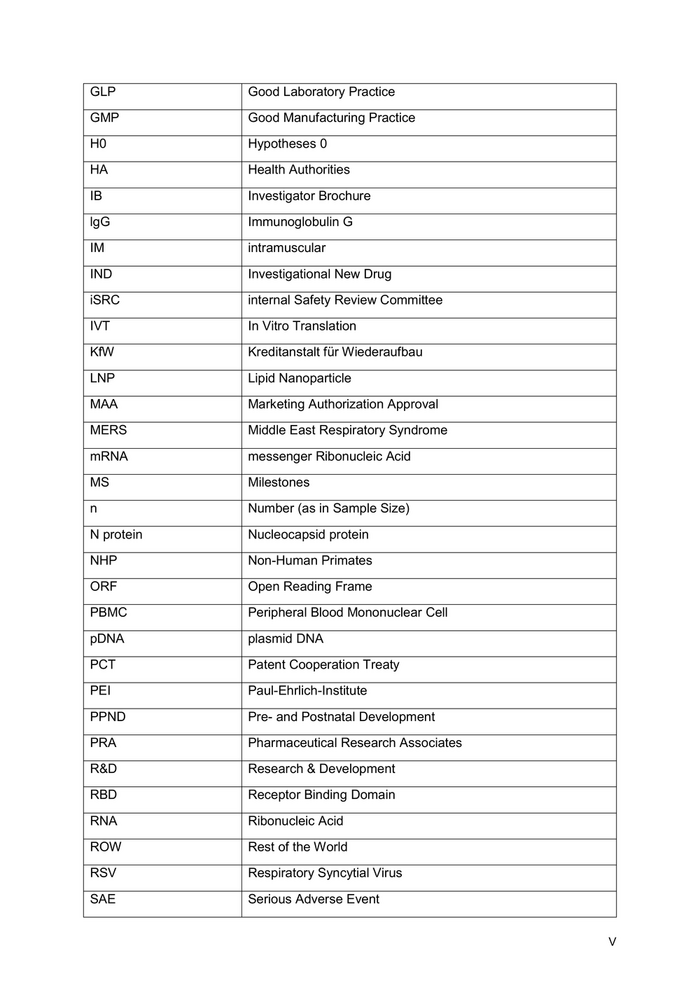
SARS Severe Acute Respiratory Syndrome S protein Spike protein US United States VNT Virus Neutralizing Titer WB Western Blot WHO World Health Organization YOA Years of Age µg Microgram VI
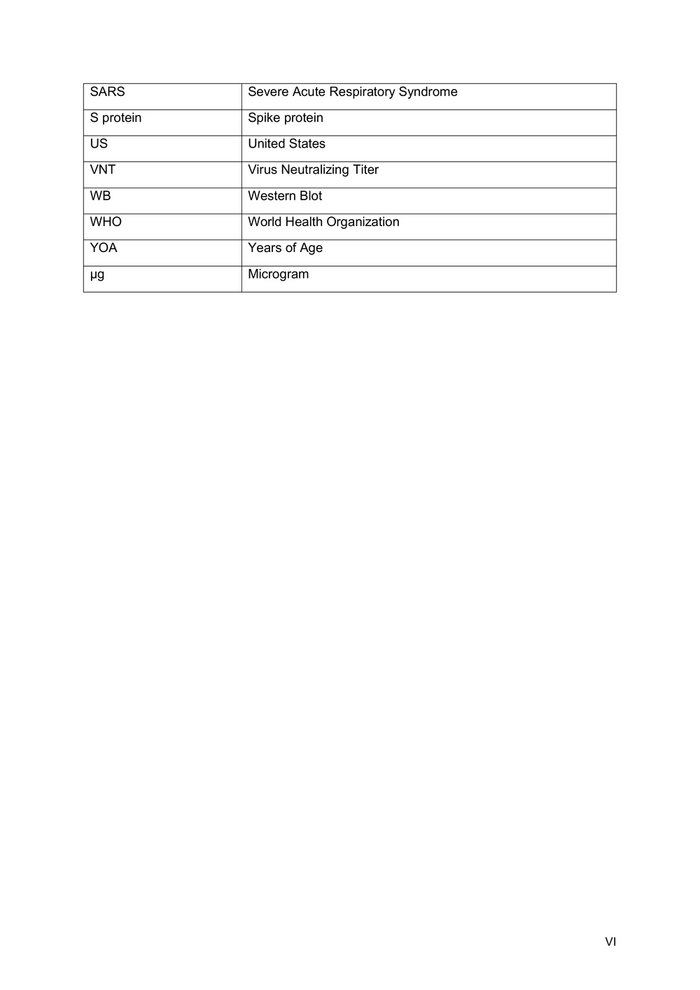
List of Tables Table 1: Executive Summary .............................................................................................. 1 Table 2: Release analytics .................................................................................................15 Table 3: Main milestones with timelines ...........................................................................23 Table 4: Comparison Table 1g vs. 10g up-scale ..............................................................33 Table 5: Materials and Slot availability .............................................................................36 Table 6: Summary on key risks, their impact and likelihood of CVnCoV development 39 Table 7: Labour/cooperation with third parties ................................................................47 Table 8: Main milestones with timelines ...........................................................................50 Table 9: Milestone volumes ...............................................................................................50 Table 10: Total costs per category ....................................................................................50 Table 11: Total costs per work package ...........................................................................50 Table 12: Batch Production Costs ....................................................................................52 Table 13: Overview of batch production ...........................................................................53 Table 14: AP 1: CMC Development & Manufacturing .......................................................55 Table 15: AP 2: Clinical Development – Dose confirmation in Elderly Phase IIa, Study 002 .......................................................................................................................................55 Table 16: Phase IIa - Elderly study ....................................................................................56 Table 17: AP 3: Clinical Development – Special populations, Phase II/III – AP 3 ...........56 Table 18: Clinical Studies Special Populations ................................................................56 Table 19: AP 4: Clinical Development – Efficacy Phase IIb/III, Study 004 and Concomitant Vaccination Study 010 .................................................................................57 Table 20: Phase IIb/III Investigator Fees and other costs corresponding costs ............58 Table 21: AP 5: Clinical Development – non interventional study ..................................58 Table 22: Additional non-interventional study .................................................................58 Table 23: AP 6: Regulatory Affairs – interactions with regulatory authorities ...............58 Table 24: AP 7: Manufacturing for Clinical Development – Risk mitigation and stockpiling for Phase IIb/III ................................................................................................59 Table 25: AP 8: Preclinical Development: Fulfillment of regulatory requirements for the marketing authorization (MAA, marketing authorization application) for the market entry in the EU ....................................................................................................................60 Table 26: AP9: Production at risk......................................................................................61 Table 27: Ramp-up of production capacity ......................................................................62 List of Figures Figure 1: Timeline of major activities until Marketing Authorization Application (MAA) approval by EMA. ................................................................................................................ 4 Figure 2: High level clinical development plan for CVnCoV for Phase I to III studies (trials 001-010). .................................................................................................................... 5 Figure 3: SARS-CoV-2 mRNA induces high levels of humoral and cellular immune responses in mice. .............................................................................................................10 Figure 4: Manufacturing overview .....................................................................................13 Figure 5: Overview of drug substance (mRNA) production steps including analysis ..14 Figure 6: Overview of drug product production steps including analysis .....................15 Figure 7: CMC Planning 2020 ............................................................................................23 Figure 8: Flow chart for Phase IIb/III study 004. ...............................................................27 Figure 9: CureVac's risk management process ...............................................................37 Figure 10 : Work package timelines ..................................................................................51 VII

Project description 1. Introduction a. Brief description of the applying companyj/institution CureVac is a leading global clinical-stage biopharmaceutical company with 20 years of expertise in developing a new class of transformative medicines based on messenger ribonucleic acid (mRNA) that has the potential to improve the lives of people. Our vision is to revolutionize medicine and open new avenues for developing therapies by enabling the body to make its own drugs. mRNA plays a central role in cellular biology in the production of proteins in every living cell. We are the pioneers in successfully harnessing mRNAs designed to prevent infections and to treat diseases by mimicking human biology to synthesize the desired proteins. Our technology platform is based on a natural approach to optimize mRNA constructs that encode functional proteins that replace defective or missing proteins using the cell’s intrinsic translation machinery. Our current product portfolio includes clinical and preclinical candidates across multiple disease indications in oncology, prophylactic vaccines and protein therapy. Our lead clinical programs, CV8102 for the treatment of four types of solid tumors and CV7202 for potential vaccination against rabies, have generated promising early efficacy and safety results in clinical trials. Furthermore, we have rapidliy advanced our mRNA vaccine candidate against coronavirus (SARS-CoV-2) through preclinical studies. We initiated the first Phase 1 clinical trial on June 18, 2020, upon the granting of the permission by the German Health Authority Paul-Ehrlich-Institute (PEI) and the Belgian Federal Agency for Medicines and Health Products (FAMHP) on June 17, 2020. On June 15, 2020, the German Federal Ministry of Economics and Energy announced its commitment to invest 300 million Euros in CureVac through the Kreditanstalt für Wiederaufbau (KfW). CureVac has also entered into collaborations with multinational corporations and organizations, including Boehringer Ingelheim, Genmab, CRISPR Therapeutics, the Bill & Melinda Gates Foundation, the Coalition for Epidemic Preparedness Innovations (CEPI) and others. The company is headquartered in Tübingen, Germany with sites in Frankfurt and Boston, USA. b. Executive Summary in tabular form Table 1: Executive Summary Applicant CureVac AG Friedrich-Miescher-Str. 15 72076 Tübingen Germany Project Manager: (companyjinstitution, project manager) Vaccine technology (max. Lipid nanoparticle (LNP) formulated mRNA consisting of 140 characters) sequence optimized, mRNA using naturally occurring nucleotides. Target Antigen (max. 140 Full length spike SARS-CoV-2 virus antigen with 2 proline amino characters) acid substitutions (K986P; V987P).

Status of preclinical Preclinical development program including immunogenicity development, (max. 100 characterization in vitro and in rodent models, GLP-repeat-dose words) toxicology in rats enabling approval and start of first in human trial (Phase 1, CVnCoV-001) in June 2020. Further non-clinical development to support clinical development clinical development and marketing authorization ongoing including mitigation strategy to provide a 2"d generation vaccine. Main milestones (MS) with timelines CureVac’s SARS-CoV-2 project aims to provide a safe and effective vaccine to prevent diseases induced by SARS-CoV-2 (COVID-19). The clinical candidate CVnCoV (CureVac novel Coronavirus vaccine) is an mRNA-based severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine encapsulated in lipid nanoparticles (LNPs) for intramuscular (IM) injection. The preclinical and analytical work of CVnCoV was successfully conducted to enable approval of a first in human (FIH) trial in Germany and Belgium (CVnCoV-001) by PEI and FAMHP, respectively. GMP (Good Manufacturing Practice) manufacturing supplied sufficient material to conduct the initial development program. Supply of GMP material for the early clinical phases, Phase 1 and 2 has been secured and supply for Phase 3 as well as initial market supply in 2021 is currently secured. An accelerated interaction with the COVID-19 European Medicines Agency (EMA) pandemic task force (COVID-ETF) is planned by August/September 2020 to reach alignment on the path forward to gain (conditional) approval by EMA as soon as feasible. Objective is to achieve an early approval via the conditional approval pathway of EMA in early 2021 followed by approval during second half of 2021. In scope are all age groups and at risk population including elderly as well as special populations, i.e. subjects with comorbidities, immunocompromised persons, pregnant women as well as the pediatric population. A further main objective is to secure expanded GMP manufacturing to be able to protect a large number of the population in Germany, Europe but also worldwide. As this is a novel virus against which currently no efficacious prophylactic vaccine exists, CureVac is including a targeted risk mitigation program to provide an improved vaccine in case of limitation in safety and/or efficacy of the current clinical candidate as fast follow-on product potentially entering into clinical trials by end of 2020. A further major objective is to increase 2

and reserve required production capacities to ensure sufficient and sustainable supply beyond 2021. Figure 1 shows the timeline of the major activities planned until approval by EMA. Details on work packages and timelines can be found in the respective sections of this application and in the Milestone Planning (Annex 1). 3